Abstract
A number of studies have shown that the motor actions of one individual can affect the attention of an observer. In one notable example, “social inhibition of return,” observers are relatively slow to initiate a response to a location where another individual has just responded. In the present article we examine the degree to which this phenomenon can be considered a social effect. We find that unlike the related social, or “joint,” Simon effect, social inhibition of return is not influenced by competitive versus cooperative interaction, nor by live versus recorded interaction. We do find however that co-actors need to turn-take in order for the effect to occur. Thus, so-called “social” inhibition of return only reaches a minimal threshold to be considered a social phenomenon.
Similar content being viewed by others
Introduction
The individual mind in isolation has long been the object of study for cognitive science. Even when social cognitive processes have been the focus of the field, many paradigms have persisted in using artificially generated social stimuli, which are then presented to participants in a solitary experimental task. This method has led to many theoretical developments of social processes, with the benefit of high levels of experimental control. However, in the past decade or so, a number of researchers have begun to examine cognitive processes when individuals interact alongside others (see Cole, Skarratt, & Kuhn, 2015, for review). A central issue within this context has been the examination of mechanisms that co-represent other people’s actions or tasks, and in particular how action observation influences an observer’s action planning (Atmaca, Sebanz, Prinz, & Knoblich, 2008; Atmaca, Sebanz, & Knoblich, 2011; Prinz & Sebanz, 2003; Tsai, Sebanz, & Knoblich, 2011).
Perhaps the most notable is the “joint” or “social” Simon effect, first described by Sebanz, Knoblich and Prinz (2003). In the basic (i.e., non-social) Simon paradigm (e.g. Craft & Simon, 1970) participants make one of two possible responses to a feature of a stimulus (for example color) that is presented either on the left or right side of a display. Characteristically, responses are facilitated when the spatial dimension of the stimulus is congruent with that of the response and slowed when these are incongruent. For instance, if a participant is required to discriminate the color of one of two possible targets by pressing a left or right button, a red target requiring a left response will be discriminated more rapidly if it happens to be presented on the left side of the display. The effect is not present if the participant is required to detect the presence of a target in which they press one button only, as in a Go/No-go task. That is, participants will not be quicker to detect the presence of the red target with a left response when it happens to occur on the left. However, the effect is reinstated if the participant shares the task with another individual sitting to their side who responds to the presence of the other target with their right hand (Sebanz, et al., 2003).
In light of the prevailing view that the classic Simon task represents the correspondence of a stimulus and planned action (Hommel, 1993; Prinz, 1997), Sebanz et al. argued that the joint action version of the effect demonstrates that people co-represent the action and task plans of co-acting individuals. Although a number of studies have since replicated and extended Sebanz et al.’s findings (Liepelt, Wenke, & Fischer, 2013; Welsh, 2009; Sebanz, Knoblich, Prinz, & Wascher, 2006; Hommel, Colzato, & van den Wildenberg, 2009; Tsai, Kuo, Hung, & Tzeng, 2008; Ruys & Aarts, 2010; Welsh, Higgins, Ray, & Weeks, 2007; Vlainic, Liepelt, Colzato, Prinz, & Hommel, 2010; Tsai & Brass, 2007; Müller et al., 2011a, b), a central issue is whether the social interaction between participants is a necessary condition of the effect. In this context, Dolk, Hommel, Prinz and Liepelt (2013) demonstrated that the joint Simon effect can be elicited when the co-actor is replaced by a stationary non-biological object that merely acts as an attention capturing cue (that induces a “referential code”; Hommel, 1993). This suggests that this particular joint effect is not actually caused by the co-representation of action, nor is it necessitated by a social interaction between two human participants. One does have to add however, that inducing the effect when no partner is present does not necessarily mean that action co-representation is not occurring when a partner is present.
A further joint action effect has also received considerable attention. In the “social inhibition of return” paradigm (IOR; e.g., Atkinson, Simpson, Skarratt, & Cole, 2014; Cole, Skarratt, & Billing, 2012; Hayes, Hansen & Elliott, 2010; Ondobaka, de Lange, Newman-Norlund, Wiemers & Bekkering, 2012; Skarratt, Cole & Kingstone, 2010; Welsh, Elliot, Anson, Dhillon, & Weeks, et al., 2005; Welsh, Lyons, Weeks, Anson, & Chua, et al., 2007; Welsh, McDougall & Weeks, 2009; Welsh, Ray, Weeks, Dewey, & Elliott, 2009), participants sit opposite each other and take turns to reach to one of two targets presented to the left and right on a flat workspace located between them. Results show that the reaching responses of one participant influence the reaching responses of their co-actor. Specifically, initiation of responses is quicker when directed to a different side of the display to that which the co-actor just responded.
Debate currently surrounds which mechanisms give rise to social IOR. The very name given to the phenomenon suggests that, for some authors, the effect is due to inhibitory mechanisms that follow when attention has been shifted to a location, i.e., IOR (Posner & Cohen, 1984). In the basic (non-social) IOR paradigm, participants are required to detect/discriminate a target that appears either at a “cued” or non-cued location. Typical results show that reaction times (RTs) are relatively long when targets occur at locations that were cued more than approximately 350 ms previously. With respect to social IOR, it has been argued (e.g., Doneva, Atkinson, Skarratt, & Cole, 2017) that the observation of a co-actor’s arm reach acts as such a cue that shifts attention to the reached-to location. As with the joint Simon effect, a central question that has not yet been examined is whether “social” IOR requires a social interaction between two human participants or whether low-level transient cues that shift visual attention are sufficient to produce the phenomenon. Support for the attention explanation has come from work showing that the effect occurs when a co-actor performs a very different action to that of the participant but, importantly, one that shifts attention to the target location (Atkinson et al. 2014; Doneva & Cole, 2014; Doneva et al., 2017). However, Ondobaka et al. (2012) have provided evidence that the effect is modulated by the goals and intentions of the co-actors, an effect that concurs more with a social account than the attentional account (but see Cole et al., 2012).
There are a number of parallels between the joint Simon effect and social IOR. Both are, by definition, joint action effects and both may or may not involve mechanisms that co-represent the action plans of an observed individual taking part in a social interaction. As such, a number of studies have attempted to assess how social the “social” Simon effect actually is by examining the nature of the interaction between the participants and its contribution to the effect. For instance, modulations of the phenomenon have been elicited by the absence versus presence of the co-actor in the same room (Welsh, Higgins et al., 2007, Welsh, Lyons et al., 2007), cooperative versus a competitive setting (Ruys & Aarts, 2010), the quality of the relationship with the co-actor (Hommel et al., 2009), biological versus non-biological action perception (Tsai & Brass, 2007), and the perceived intentionality of a co-actor (Tsai et al., 2008). Particularly strong evidence for the “socialness” of this joint action effect has come from studies showing that social identity and group membership can influence its magnitude (McClung, Jentzsch, & Reicher, 2013; Müller et al., 2011a, b). Although these studies do suggest that the joint Simon effect is indeed a social phenomenon, the Dolk et al. (2013) findings (see above) do show that biological agency is not necessary for the effect to occur. Indeed, Dolk and colleagues suggest that the alternative term, the “joint” Simon effect, replaces the more common “social” designation.
In the present article, we examined whether social IOR can also be considered a social effect. Whilst there are obvious differences between the joint Simon and social IOR paradigms, there are also similarities. For instance, both present participants with salient visual “transients” resulting from a partner’s actions. These actions could cue attention and induce automatic motor preparation processes that are resistant to top-down social manipulations – manipulations that may influence the joint Simon effect. On the other hand, the nature of the social interaction between the participants may influence the processing of these cues in the social IOR paradigm, such that even salient reaching responses can be processed differently as a result of the social interaction taking place. In three experiments we varied the nature of the interaction between participants who undertook the basic social IOR procedure. Specifically, we manipulated competitive versus cooperative interaction (Experiment 1), live physical interaction (i.e., the standard paradigm) versus remote and recorded interactions (Experiment 2), and regular turn-taking versus independent turn-taking (Experiment 3). Finally, “social” is a higher-level construct and can therefore be difficult to define. The present paper’s definition is a manipulation in which the stimuli inducing the basic social IOR effect does not change at all, as in Experiment 1, or is minimized, as in Experiment 2. Only the immediate interaction context between coactors is changed.
Experiment 1
In Experiment 1, pairs of participants performed a standard social IOR task either competitively against each other or cooperatively against the other pairs in the experiment. The interactive social context with which one participant viewed their partner thus differed, yet throughout, identical goal-directed actions were observed. In the “cooperation” condition, the pairs of co-actors were told that they should consider themselves a team and that the pair with the shortest mean RT would receive a cash prize. In the “competition” condition, the pairs were instructed to compete against each other to win the cash prize, which would be awarded to the fastest individual in each pair. This therefore paralleled the cooperative/competitive manipulations used by Ruys and Aarts (2010) in the joint Simon effect. These authors found that both manipulations increased the magnitude of the effect. Moreover, in a second experiment they showed that participants who scored highly in a test of social attention, showed a greater joint Simon effect than those with low scores. Finally, in a competitive scenario, those with low social attention scores showed a greater joint Simon effect than in a neutral interaction (the authors did not test whether a cooperative scenario interacted in the same way with social attention). One explanation for these findings is that the demands of competitive (and potentially cooperative) interaction increase social attention, which is itself associated with co-representing another’s action or task, including during the joint Simon paradigm (Dolk et al., 2013).
Method
Participants
Participants were 36 right-handed undergraduate students aged 18–31 years recruited via a University of Essex participant pool. They were given £10 for approximately 40 minutes of participation, in addition to the opportunity to win the cash prizes. All stated that they were right-handed, had normal or corrected-to-normal vision and were naïve to the purposes of the experiment. We conducted a sample size analysis using a simulation in the R environment. This aimed to ensure our sample had adequate power to detect a conservative social IOR effect and to detect an interaction where, in a 2 × 2 within-participants design (see Design), one repeated measures factor obtained this effect and in another it was absent. As such, we used a mean effect size calculated by Cole, Atkinson, D’Souza, Welsh, and Skarratt (2017) in a recent cross-study meta-analysis of ten social IOR experiments. We used the eight experiments that recorded response times only. Using these data we estimated a population standard deviation of σ = 50 ms, a mean population response time of μ = 350 ms, and a mean social IOR effect of 14 ms in the population. Bivariate correlations between repeated measures ranged from r = .66 to .99, so were estimated to be ρ = .85 across all conditions in the population. Correlations between pairs ranged from 0.07 to 0.57 and were estimated to be ρ = 0.3 in all conditions. We then calculated the power with which such an effect could be detected with α = 0.05, using linear mixed-effects regression (LMER; see Results). The simulations revealed that with 36 participants, a 14-ms effect size could be detected in the target location condition, with power equal to .86. In the case of an interaction, where a social IOR effect was revealed in either the cooperation or completion condition only, the present sample could detect the interaction with a power of .87. The simulations therefore demonstrated that the present sample size was sufficient to detect a typically observed social IOR effect and an interaction where this was observed in only one of the two conditions.
Stimuli and apparatus
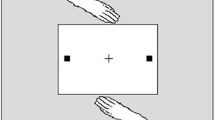
Figure 1 depicts the response environment used in Experiment 1. A black fixation cross (0.4 cd/m2) was displayed centrally against a uniformly white (73.7 cd/m2) background. Response positions were black squares (0.4 cd/m2) and grouping objects were dark grey (28 cd/m2). Placeholder squares became targets by illuminating to white (73.7 cd/m2). There were four response position squares, placed in pairs to the left and right of fixation at a distance of 175 mm as measured from their middle. A rectangle surrounded each pair of squares, measuring 140 mm long and 22 mm wide. This created two response positions for each participant. Each had one located to the left and one two the right, positioned to their own side of the display. The experiment employed custom-programmed software presented on a Pentium-enabled PC. This software controlled the presentation of stimuli and measured the latency of responses. A 22-in. LCD monitor was embedded into the table. Participants rested upon a “home” button between trials. Mean RT was recorded when these buttons were released, following the initiation of an action on each trial. The monitor was placed 240 mm from each participant’s home button and 740 mm from the floor. All the present experiments partly restricted the visibility of a co-actor’s action via the positioning of two physical barriers (see Fig. 1; Skarratt et al., 2010; Welsh et al., 2009). The central aperture of 12o allowed each participant to view the initiation of a co-actor’s response but not the presentation of their target, nor peripheral transient visual events brought about by the arm movement. This aspect of the stimuli eliminates some of the large peripheral transients that could generate social IOR (see Skarratt et al., 2010; Welsh, Higgins et al., 2007, Welsh, Lyons et al., 2007).
Design and procedure
A 2 × 2 fully within-participants design was employed. The first factor was target location relative to the previous trial (same and different) and the second was the cooperation and competition manipulation. The levels of the target location factor were presented randomly within blocks whilst the second factor was blocked and presentation order counterbalanced. The central fixation cross was present for an entire block and 350 ms elapsed between response completion and the next target occurring. Participants were asked to fixate on the cross until they were required to make their response, during which they were instructed to fixate the target. They were also instructed to initiate all reaching movements to targets as rapidly but as accurately as they were able. Each experimental trial began with the illumination of the target square for 100 ms. If no response was made after 2,000 ms, the next trial began. In the cooperation condition, the following instructions were presented: “You are competing as a pair, or a ‘team,’ against the other 17 pairs that will take part in this study: The four pairs who have the fastest average response time will win £5 each at the end of the study. A penalty of 10 ms will be added for each error made. It is thus important to be both fast and accurate together throughout.” In the competition condition, participants received the following instructions: “You are competing against your partner. The person who has the fastest average response time will win £2. A penalty of 10 ms will be added for each error made. It is thus important to be both the fastest and most accurate throughout.” Finally, no target appeared in the same left or right location more than four times sequentially and each block consisted of 209 trials. Thus, there were 418 trials in total, i.e., two blocks, one cooperation, one competition. Because social IOR is an effect based on responses that follow a previous response, the first trial of each block did not form part of the analysis.
Results and discussion
RTs less than 100 ms were removed as anticipation errors and those above 1,000 ms were omitted as inattention errors. In addition, RTs greater or less than two standard deviations from the mean were removed. This resulted in the removal of 6.3% of the data. No localization errors were made in any of the current experiments. In addition, one participant failed to register any data due to technical reasons and was omitted from the analysis.
The data exhibited a clear hierarchical structure, with each trial response (level 1), being nested within a participant (level 2), who were nested within a pair of participants (level 3). This hierarchical structure introduced the likelihood that the residuals of each participant’s response were correlated with that of their partner, such that these residuals were not independent. The data were therefore analyzed with LMER using the R package nlme (Pinheiro, Bates, DebRoy, & Sarkar, 2014). Data for each trial were not aggregated and were entered into the analysis with participant and pair as random intercepts in the model. This allowed for the control of variance associated not only with each participant, but also each pair. The hypotheses of Experiment 1 were tested by specifying a random intercept model, which included the two levels of social condition (cooperation, competition) and the two levels of the target location condition (same, different) as fixed effects. Random intercepts were specified for target location and social condition, nested within participants and pairs. We report the results of type III Wald F tests with Kenward-Roger degrees of freedom approximation for each fixed effect and interaction. Means are shown in Fig. 2. In addition to the frequentialist hypothesis tests of the effects of social condition, location condition, and the interaction between these factors, we computed Bayes factors for each effect using the R package BayesFactor (Morey, Rouder, & Jamil, 2015). Bayes factors are calculated for each model using a mixture of g priors centered around zero (Liang et al., 2008). We report the Bayes factor of each fixed main effect relative to the baseline model, which included only the random intercepts for participant and pair. Finally, we computed the Bayes factors comparing the additive main effects model with the model that included both main effects and the interaction term (e.g., Wagenmakers, 2007), and interpreted the strength of evidence indicated according to Rafferty (1995). We therefore evaluated the relative evidence in favor of the influence of social manipulations on the social IOR effect, against the evidence for the alternative, where the effect was independent of these manipulations.
The results revealed an effect of target location (F(1, 6796) = 468.25, p < .001, BF10 = 2.80 × 1096) and an effect of social condition (F(1, 6796) = 25.38, p <.001, BF10 = 3538.20). However, there was no interaction between the fixed effects (F(1, 6796) = 1.77 p = .18, BF10 = 0.09). The Bayes factor comparing the additive main effects model with the alternative model, which included the interaction term, revealed strong evidence in favor of the main effects model only. RTs were longer in the same location trials relative to the different location trials across both competition and cooperation levels of social condition (M = 36.19, SE = 2.23, d = 1.40).
Overall, these results show a clear social IOR effect; participants are slower to initiate a reaching response to the same side of the display that their co-actor just reached to. However, the size of the effect was not influenced by a cooperative or competitive interaction between participants. This in turn suggests that the mechanism that gives rise to this phenomenon is not influenced by interactions that promote attention to another’s action or encourage shared action plans. Such a finding contrasts with previous reports concerning the joint Simon effect (Hommel, et al., 2009; Ruys & Aarts, 2010). Instead, the current data are consistent with the proposal that the locations responded to by another are inhibited independently of social context.
Experiment 2
Perhaps the most direct way of examining whether an effect is “social” is to assess whether it is modulated by the presence of a real biological agent participating in a live interaction. For both social attention and joint action, the experience of, or belief that, a live interaction is occurring has been shown to influence performance. For instance, live actors provide effective stimuli to induce the gaze-cueing effect (Cole, Smith, & Atkinson, 2015; Lachat, Conty, Hugueville, & George, 2012). Moreover, even when gaze stimuli are not live, there is some evidence that gaze cueing can be modulated by whether participants think the gazing agent can “see” the cued targets (Teufel, Alexis, Clayton, & Davis, 2010; but see Cole, Smith, & Atkinson, 2015, and Cole, Atkinson, D’Souza, & Smith, 2017). Thus, the attribution of relevant mental states to others may influence behaviors where attention is shifted in line with that other person. Similar investigations concerning joint action effects, such as the joint Simon effect, have revealed that the mere belief that a live interaction is taking place is sufficient to elicit effects even when participants actually act alone (Welsh, Higgins et al., 2007, Welsh, Lyons et al., 2007).
The variable of interest in the present experiment was again the social interaction between participants, all of which were tested in three experimental conditions. In one, participants performed a standard social IOR procedure in which they sat opposite each other and responded to targets on a shared workspace. In the other two conditions, the procedure was performed with co-actors visible through a widescreen monitor. In one of these, participants viewed a live video feed of their partner performing the task, and in the other, they viewed a synchronized recording of a practice block that all participants took part in prior to the experimental procedure. No deception was used to manipulate participants’ experience of the task and they were always explicitly informed when they were acting in the live or recorded conditions, respectively.
If the presence of a biological agent is required to induce social IOR (in contrast to an animated partner) then the effect should be observed only in the condition where participants sit physically opposite. Furthermore, if the animated partner fails to induce social IOR because there is no true interaction between participants, then the effect should occur with a physically present co-actor, and a remote but live co-actor, but not a recorded co-actor. Moreover, the transient, or low-level, visual input for both the live and recorded co-actors was identical; however, participants possessed the top-down knowledge that the procedure was not a true interaction with a present partner.
Method
Participants
Thirty-six participants took part (18 females, 18–38 years old) in return for a course credit or monetary payment at the standard rate hourly rate of the University of Essex. None of the participants completed the previous experiment or any other social IOR study. All were right-handed and reported normal or corrected-to-normal vision. Again we conducted a sample size analysis using simulation methods and using the same population data estimated from the Cole et al. (2017) meta-analysis data. This found that in the case where two social interaction conditions yielded a social IOR effect, an interaction could be detected with a power of .87. Alternatively, if a social IOR effect was observed in only one social interaction condition, an interaction effect could be detected with a power of .92. Again, the present sample of 36 participants was thought to be adequate to detect interaction effects typical of social IOR paradigms.
Stimuli and apparatus
Figure 3 depicts the apparatus employed in Experiment 2. All stimuli in the live and recorded video conditions were presented on a table measuring 2,450 × 650 mm, where the surface was 670 mm from the floor. On the table were affixed four light boxes and two switch boxes (120 mm in diameter). The light boxes contained red light-emitting diodes (4 mm in diameter), which illuminated for a participant’s target. Participants could see their partner through a widescreen display measuring 620 × 430 mm. Material was affixed to the sides of each monitor, leaving a central visible strip of 12o in width, which matched the dimensions of Skarratt et al. (2010). A central fixation cross was drawn and positioned directly under the bottom edge of each monitor, measuring 30 mm along each line. The image of each partner was recorded or fed into the monitors live, using Panasonic digital cameras, mounted on desktop tripods. These tripods were placed behind each participant’s monitor in a central space, measuring 110 mm, with the cameras sitting 690 mm from the table surface and tilted in order to frame the appropriate partner in the participant’s displays. Material was affixed to the tripods to occlude any visibility for taller participants above the top of the display screens. The light boxes were horizontally positioned, such that the central diode was 440 mm from central fixation and two were placed left and right under each participant’s display. The button boxes were positioned 590 mm from the central fixation point. The four light boxes and home buttons were placed on either side of the barrier, matching the dimensions used in the live and recorded video conditions. An experimental procedure written in Superlab (Cedrus systems) and running on an Apple MacBook Pro controlled the presentation of stimuli and recording of RTs.
Design and procedure
A 2 × 3 fully repeated measures design was employed. The first factor was again target location relative to the previous trial (same and different). This was presented randomly throughout the three experimental blocks. The second factor (social condition) had three levels. The “physical” condition was a baseline measure, where participants completed a basic social IOR procedure, sitting opposite to their co-actor. The “live” condition presented the co-actor on the monitor live as they completed the procedure via the cameras. In the “recorded” condition a participant’s co-actor was recorded during the practice trials and this was presented via the monitor. The levels of social condition were blocked and counterbalanced among participants. Participants responded by performing a reaching action and touching the central diode of the target that illuminated. There were 627 trials in total, i.e., three blocks of 209. Prior to the three experimental blocks, participants completed a full practice block, which was recorded by the cameras.
Results and discussion
Of the data, 6.72% were omitted according to the constraints applied in Experiment 1. One participant was omitted following a failure to record their responses. Means are shown in Fig. 4. The hypotheses of Experiment 2 were again tested using an LMER with three levels of social condition (physical, live, and recorded) and two levels of target location (same, different) specified as fixed effects and pairs, and participants as random intercepts. This revealed a main effect of location (F(1, 7098) = 4.31, p = .038, BF01 = 1.25 × 10516) and a main effect of social condition (F(2, 7098) = 66.06, p < .001, BF01 = 4.21 × 1013). There was, however, no interaction between social condition and location (F(2, 7098) = 0.58, p =0.50, BF01 = 0.007), where the Bayes factor can be interpreted as very strong evidence in favor of the hypothesis that there is an additive main effects model over an interaction model. Mean RTs were longer in the same-location trials than in the different-location trials across the physical, live, and recorded levels of social condition (M = 2.67, SE = 1.25, d = 0.11). Thus, the data show the presence of a social IOR effect across all three social conditions. Overall, these findings indicate that the effect is not influenced by the physical presence of a co-actor or the visual feedback of their responses being presented to participants live.
The present data confirm that two co-actors need not be physically present for social IOR to occur. As with Experiment 1, the results support the view that social IOR is not modulated by top-down social influences, requiring only the observation of another’s arm reach prior to the presentation of a response target. In addition, it is not necessary for participants to witness biological motion live. These findings are also consistent with a number of reports from the mimicry and attention literature, that mere observation of another’s action can elicit automatic motor plans or attention shifting in the observer (e.g., Brass et al., 2000; Shi, Weng, He, & Jiang, 2010). Typically, these studies do not present the observed action stimuli as part of a live interaction, yet robust imitation effects and cueing effects have been observed. The present results show that such recorded stimuli do not influence the presence of social IOR.
Experiment 3
The joint actions that take place within the social IOR paradigm may be thought of as reaching the minimal threshold for social interaction. Apart from the fact that co-actors sit opposite each other, the most social aspect of the paradigm is in the fact that co-actors turn-take: they are required to complete their response and wait until their partner completes her response. As such, the rhythm of this sequence and the expectation of a partner’s actions may influence a participant’s tendency to attend to them. In Experiment 3 we examined the degree to which social IOR occurs when participants take turns (i.e., as in the standard paradigm) compared with when they are not required to follow a regular turn-based sequence. In this latter condition, targets appeared at random intervals rather than rhythmically. For instance, sometimes both co-actors’ targets appeared simultaneously whilst at other times one co-actor would not be presented with any targets for a few seconds/trials. Critically, however, on a subset of trials the interval between two consecutive responses was the same as in the standard (turn-taking) paradigm. That is, 350 ms elapsed between the completion of a response by co-actor A and the target presented on the next trial (to co-actor B). If social IOR only occurs when co-actors are required to attend to each other’s actions (i.e., when they turn-take), the effect will have reached the minimal definition to be considered a social phenomenon. If, by contrast, social IOR occurs irrespective of whether co-actors regularly take turns, we posit that the effect cannot be considered a social effect since it will have been shown to occur when there is no interaction between co-actors.
Method
Participants
Thirty-six right-handed participants (17 females) aged 18–29 years were recruited via the University of Essex human participant pool in return for either a course credit or financial remuneration. Participants had not previously undertaken a study in this paradigm and reported normal or corrected-to-normal vision. The participant sample size was thought to be acceptable for detecting an interaction effect at power > .80, following the simulation models employed in Experiment 1.
Apparatus
The experiment employed the same apparatus used in Experiment 1.
Stimuli
Stimuli were the same in all respects as those presented in Experiment 1.
Design and procedure
The study used a 2 × 2 fully within-participants design. The first factor again manipulated target location relative to the previous trial (same and different) and the second manipulated turn-taking (turn-taking, no turn-taking). The levels of target location were presented randomly within-block and the two turn-taking conditions were blocked. Four blocks of trials were presented; one turn-taking block, and three no-turn-taking blocks. When targets are presented at random intervals to both co-actors (i.e., they are not turn-taking), this necessarily means that there are not many of the critical trials to analyze. Recall that the critical trials are those in which one of the co-actors completes their response and 350 ms later their partner’s target appears. Because this can only happen occasionally, three non-turn-taking blocks were required to increase trial number. These three blocks comprised a total of 627 trials. On 208 of these the interval between response completions by co-actor A and the target presentation for co-actor B was 350 ms, i.e., the same interval that occurs in the turn-taking block. Of the remaining 419 trials, 314 trials presented targets to both participants simultaneously and 105 presented a single target to one participant; however, this followed their own previous response. These constraints resulted in an equal amount of trials performed individually and simultaneously across the three blocks. Crucially, in the no-turn-taking blocks could participants not predict either the occurrence of their own or partner’s response as in a turn-based procedure. For the turn-taking condition, all aspects of the procedure were as in the standard paradigm that generates the basic social IOR effect. All blocks of the experiment were counterbalanced according to a Latin square design. Each block was pre-randomized to ensure that all trials in this condition met the constraints previously described.
Results and discussion
Four participants were removed from the analysis due to failure of responses to be recorded. Because of this, we conducted sample size simulations based upon 32 participants. These revealed that both main social IOR and interaction effects could be detected at a power of .82, indicating adequate power levels for further analysis. Of the remaining data, 3.9% were omitted according to the constraints applied in Experiments 1 and 2. Figure 5 displays the mean RTs across the four conditions.
Once again LMER was employed, specifying target location (same and different) and task condition (turn-taking, no turn-taking) as fixed effects and participant nested in pair as random intercepts. There was a main effect of target location (F(1, 5205) = 19.26 p < .001, BF10 = 154.27). There was no effect of task condition (F(1, 5205) = 0.66, p > .10, BF10 = 0.14); however, there was a significant interaction between target location and task condition (F(1, 5205) = 6.45, p = .01, BF10 = 0.85). A significant social IOR effect was observed in the turn-taking condition, (t(3017) = 4.96, p < .001, M = 8.75, SE= 2.17, d = .46), but no such effect was present in the no-turn-taking condition, (t(3017) = 2.04, p >.10, M = 2.05, SE = 2.92, d = .07). A note of caution in the interpretation of these results pertains to the Bayes factor, which indicates weak/anecdotal evidence in favor of the hypothesis that there were additive main effects only. In Bayesian terms, therefore, the presence of an interaction in this model is inconclusive.
Overall these data show that social IOR is modulated by whether the observed and executed reaching action is part of a regular, turn-based task. Specifically, it was found that only in the regular turn task was social IOR present. The results suggest that the same visual percept of another’s response may receive different inhibitory tagging, depending upon the task or interactive context within which it is observed.
General discussion
A number of studies have shown that participants are slower to respond to a location that was previously reached to by another individual. The current work, in line with other joint action studies, investigated the role of participant interactions in this “social IOR” effect (Hommel, et al., 2009; Risko, Laidlaw, Freeth, Foulsham, & Kingstone, 2012; Ruys & Aarts, 2010; Skarratt, Cole, & Kuhn, 2012). It was found that although the phenomenon is not affected by whether actions are cooperative or competitive (Experiment 1), nor whether or not they are performed as part of a live interaction (Experiment 2), the regular turn-based structure of the paradigm is necessary to observe the effect (Experiment 3). Furthermore, participants’ overall responses (i.e., across both target locations) were faster when they were behaving competitively (Experiment 1), and when the procedure was conducted live and in person (Experiment 2). These main effects indicate that the social manipulations employed were powerful enough to elicit RT differences across their various conditions. Nonetheless, only in Experiment 3 did these manipulations influence the social IOR effect.
Experiments 1 and 2 contrast with the findings of other studies in the joint action literature, which employed similar manipulations of social context. Both competitive/cooperative co-actor relationships (Experiment 1), as well as beliefs about the agency of the co-actor (Experiment 2), are sufficient to modulate the joint Simon effect (Ruys & Aarts, 2010; Tsai, et al., 2008). The different results across the joint Simon and social IOR paradigms may be explained by the fact that, in the latter, unlike the former, participants clearly see the large response made by a partner’s actions on a trial-by-trial basis. Thus, shifting another’s attention by a large transient signal could override any co-representation effects that could occur in the current paradigm. Indeed, participants may still co-represent the action of another, in line with several findings that suggest observed actions can create motor representations that affect future performance (e.g., Brass, et al., 2000; Kilner et al., 2004). However, in the current work, the gaze and action direction of a partner’s response also orients attention to a particular area of visual space. In support of the presence of these two distinct processes, Experiments 1 and 2 found that actions performed on both the same and different locations as partners were affected by the social condition. Thus, it may be that orienting processes are independent of co-representation mechanisms and both may affect response latencies during joint action. These questions could foster further work to determine what components of social interactions independently affect attention to another’s movements and how action co-representation determines the planning of future responses.
The present results also suggest that social IOR is abolished only when co-actors cannot or do not need to attend to their partner’s responses. In Experiment 3, the lack of social IOR in the no-turn-taking condition may be due to participants having expended extra-cognitive resource monitoring for their own targets, the presentation of which could not be predicted with respect to a previous trial. In the case of the current experiment, there was no indication whether or when a target was going to be presented at any given time. This temporal unpredictability would have required additional attentional resources from the participants in order to detect the target. Conditions of high cognitive load (e.g., perceptual load or task difficulty) are known to reduce or abolish orienting effects, including IOR (Lavie, Hirst, de Fockert, & Viding, 2004). In the turn-taking condition by contrast, participants are guided as to when their own target will be presented by the cessation of their partner’s turn. This has the effect of freeing their attentional resources during their partner’s turn. In addition, although monitoring their partner was not necessary to detect their own targets, participants would necessarily have to allocate their attention to their partner’s movements in order to anticipate the onset of their own trial. Furthermore, although the abolishment of social IOR in the no-turn-taking condition appears to refute the attentional-capture-by-motion-transients account of the basic effect (Cole et al., 2012), many transients that are known to induce capture fail to do so if load is high enough (e.g., Bobak & Langton, 2015).
In traditional paradigms of social attention, participants perform tasks alone to schematics or psychophysical representations of gaze and action stimuli. Typically, centrally presented social cues such as gaze and arrow symbols do not induce IOR at the same stimulus-onset asynchronies (SOAs) as exogenous cues, i.e., peripheral transients such as luminance changes (Frischen, et al., 2007b; Frischen & Tipper, 2004; McKee et al., 2007; Posner & Cohen, 1984). However, in the social IOR procedure, the centrally observed actions and head/eye gaze direction of partners does induce such inhibition. How do the present findings relate to the previous work? When compared with traditional Posner cueing paradigms the social IOR procedure presents each participant with large, central, salient motion cues generated from another’s goal-directed action. Although some gaze-cueing paradigms have now utilized moving gaze cues (Lachat, et al., 2012), the motion is not as salient as that found in the current joint action paradigm, which combines goal-directed action, gaze, and body orientation cues, all of which can cue attention (Gervais, Reed, Beall & Roberts 2010; Lindemann, Nuku, Rueschemeyer, & Bekkering, 2011). Motion onset itself is a powerful cue for attention and is known to elicit IOR (Abrams & Christ, 2003). Some studies have described IOR following gaze cues. However, the effects described emerge at much longer SOAs than classic exogenous cues. They also necessitate a central transient at fixation to reorient attention between the cue presentation and target onset (Frischen & Tipper, 2004; Frischen et al., 2007a, b). Whilst the SOAs employed in a social IOR paradigm (350 ms) would be much shorter than those used in these gaze cueing paradigms (1,200–1,600 ms), co-actors return their hands to the home button at the center of a participant’s visual field, behind the fixation point. This event may reorient attention between the cueing effect of a partner’s action and subsequent target presentation. This characteristic of the procedure may therefore be crucial to generating IOR in the current paradigm. If exogenous orienting is occurring to transient motion cues in this way, this reflexive effect may mask any subtle social effects that can emerge with the experimental control of traditional Posner cueing paradigms.
Despite the present results, other work in the gaze cueing literature has indicated that orienting to social cues may be modulated by top-down social factors (Nuku & Bekkering, 2008; Teufel, et al., 2010; Wiese, et al., 2012; but see Cole et al., 2015; Cole, Atkinson, & Smith, 2016a). A particular focus of study has been the modulation of attention to gaze based on the mental states of the gaze cue (i.e., whether they can see the target, or whether they are a human capable of having intentional mental states such as beliefs, desires, and intentions). This work has found some evidence that social attention is sensitive to such top-down beliefs. For instance, social attention has been shown to be modulated according to whether participants believe that they are interacting with another agent, in possession of mental states. The current data did not support this position. Under identical visual conditions, social factors – including whether the co-actor was a live agent, possessing mental states did not modulate social IOR.
Greater experimental control that comes at the expense of ecological validity is a commonly held assumption in psychological science. Nonetheless, there has been a call within a number of fields to seek and use methods that can combine as much experimental control as possible, whilst allowing participants to take part in procedures that more accurately reflect their everyday visual or social experience (Foulsham, Walker, & Kingstone, 2011; Kingstone et al., 2003). There is no doubt that this approach has yielded new insights for a number of fields in social cognitive psychology and neuroscience (Galantucci & Sebanz, 2009; Risko et al., 2012). One label that has been given to the use of this approach to study traditional areas of cognitive neuroscience such as attention and perception is cognitive ethology (Smilek, Birmingham, Cameron, Bischof, & Kingstone, 2006). This term implies the study of cognitive processes in humans within the environments that they typically occur. As a joint task with another participant co-actor, the social IOR method is a relatively ecologically valid paradigm compared with most visual cognition work in that it does reflect the fact that humans often make arm-reach movement in close proximity to others. This procedure has revealed that IOR – a hallmark of exogenous attentional capture, is a pervasive effect that is present following the orienting of another’s action and/or gaze and head direction. Previously, IOR following these social stimuli was thought to be distinct from low-level exogenous orienting, rarely found in traditional Posner cueing paradigms and with observations only occurring under quite specific experimental conditions. As such, social IOR might provide one further insight into the use of ecologically valid paradigms in the study of visual attention. This is that the most subtle social effects might not be revealed in the most ecologically valid social procedures. Instead, the current data suggest that in real, dynamic social interactions there are many highly salient attention-capturing events from another’s movements that are robust, pervasive, and hence difficult to abolish. The insight from this approach is therefore that under these familiar conditions, social cues such as another’s manual, goal-directed actions are highly effective in capturing visual attention across a range of social contexts.
References
Abrams, R. A., & Christ, S. E. (2003). Motion onset captures attention. Psychological Science, 14(5), 427–432.
Atkinson, M. A., Simpson, A., Skarratt, P. A., Cole, G. G. (2014). Is social inhibition of return due to action co-representation? Acta Psychol (Amst), 150, 85-93. https://doi.org/10.1016/j.actpsy.2014.04.003
Atmaca, S., Sebanz, N., Knoblich, G. (2011). The joint flanker effect: sharing tasks with real and imagined co-actors. Experimental Brain Research, 1-15.
Atmaca, S., Sebanz, N., Prinz, W., Knoblich, G. (2008). Action co-representation: The joint SNARC effect. Social neuroscience, 3(3-4), 410-420.
Bobak AK and Langton SR(2015) Working memory load disrupts gaze-cued orienting of attention. Front. Psychol. 6:1258. https://doi.org/10.3389/fpsyg.2015.01258
Brass, M., Bekkering, H., Wohlschläger, A., Prinz, W. (2000). Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain and cognition, 44, 124-143. https://doi.org/10.1006/brcg.2000.1225
Cole, G. G., Skarratt, P. A., & Billing, R-C. (2012). Do action goals mediate social inhibition of return?. Psychological Research, 76(6), 736–746.
Cole, G. G., Atkinson, M., D’Souza, A., Smith, D. (2017). Central cuing and spontaneous perspective taking. Vision, 1, 17.
Cole, G. G., Atkinson, M., Le, A., Smith, D. (2016). Do humans spontaneously take the perspective of others? Acta Psychologica, 164, 165-168.
Cole, G. G., Smith, D. T., Atkinson, M. A. (2015). Mental state attribution and the gaze cueing effect. Atten Percept Psychophys, 77(4), 1105-1115. https://doi.org/10.3758/s13414-014-0780-6
Craft, J. L., Simon, J. R. (1970). Processing symbolic information from a visual display: Interference from an irrelevant directional cue. Journal of Experimental Psychology, 83(3p1), 415.
Dolk, T., Hommel, B., Prinz, W., & Liepelt, R. (2013). The (not so) social Simon effect: A referential coding account. Journal of Experimental Psychology: Human Perception and Performance, 39(5), 1248–1260.
Doneva, S., Cole, G. G. (2014). The role of attention in a joint-action effect. PLOS One, 9, 3, e91336.
Doneva, S. P., Atkinson, M. A., Skarratt, P. A., & Cole, G. G. (2017) Action or attention in social inhibition of return?. Psychological Research, 81(1), 43–54.
Foulsham, T., Walker, E., & Kingstone, A. (2011). The where, what and when of gaze allocation in the lab and the natural environment. Vision research, 51(17), 1920-1931.
Frischen, A., Bayliss, A. P., Tipper, S. P. (2007a). Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychological Bulletin, 133(4), 694.
Frischen, A., Smilek, D., Eastwood, J. D., Tipper, S. P. (2007b). Inhibition of return in response to gaze cues: The roles of time course and fixation cue. Visual Cognition, 15(8), 881-895. https://doi.org/10.1080/13506280601112493
Frischen, A., Tipper, S. P. (2004). Orienting attention via observed gaze shift evokes longer term inhibitory effects: implications for social interactions, attention, and memory. Journal of Experimental Psychology: General, 133(4), 516.
Galantucci, B., & Sebanz, N. (2009). Joint action: current perspectives. Topics in Cognitive Science, 1(2), 255-259.
Gervais, W. M., Reed, C. L., Beall, P. M., Roberts, R. J., Jr (2010). Implied body action directs spatial attention. [Research Support, Non-U.S. Gov't]. Attention, perception & psychophysics, 72(6), 1437-1443. https://doi.org/10.3758/APP.72.6.1437
Hayes, S. J., Hansen, S., & Elliott, D. (2010). Between-person effects on attention and action: Joe and Fred revisited. Psychological Research PRPF, 74(3), 302-312.
Hommel, B. (1993). The role of attention for the Simon effect. Psychological Research, 55, 208–222.
Hommel, B., Colzato, L. S., van den Wildenberg, W. P. M. (2009). How Social Are Task Representations? Psychological Science, 20(7), 794-798. https://doi.org/10.1111/j.1467-9280.2009.02367.x
Kingstone, A., Smilek, D., Ristic, J., Kelland Friesen, C., & Eastwood, J. D. (2003). Attention, researchers! It is time to take a look at the real world. Current Directions in Psychological Science, 12(5), 176-180.
Lachat, F., Conty, L., Hugueville, L., George, N. (2012). Gaze Cueing Effect in a Face-to-Face Situation. Journal of Nonverbal Behavior, 36(3), 177-190.
Lavie, N., Hirst, A., de Fockert, J. W., Viding, E. (2004). Load theory of selective attention and cognitive control. J Exp Psychol Gen, 133(3), 339-354. https://doi.org/10.1037/0096-3445.133.3.339
Liang, F., Paulo, R., Molina, G., Clyde, M. A., Berger, J. O. (2008). Mixtures of g priors for Bayesian variable selection. Journal of the American Statistical Association, 103(481), 410-423.
Liepelt, R., Wenke, D., Fischer, R. (2013). Effects of feature integration in a hands-crossed version of the Social Simon paradigm. [Research Support, Non-U.S. Gov't]. Psychological research, 77(2), 240-248. https://doi.org/10.1007/s00426-012-0425-0
Lindemann, O., Nuku, P., Rueschemeyer, S. A., Bekkering, H. (2011). Grasping the other's attention: The role of animacy in action cueing of joint attention. Vision Research.
McClung, J. S., Jentzsch, I., Reicher, S. D. (2013). Group membership affects spontaneous mental representation: failure to represent the out-group in a joint action task. PloS one, 8(11), e79178.
McKee, D., Christie, J., Klein, R. (2007). On the uniqueness of attentional capture by uninformative gaze cues: Facilitation interacts with the Simon effect and is rarely followed by IOR. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale, 61(4), 293.
Morey, R. D., Rouder, J. N., Jamil, T. (2015). BayesFactor: Computation of Bayes factors for common designs. R package version 0.9, 9, 2014.
Müller, B. C., Brass, M., Kühn, S., Tsai, C.-C., Nieuwboer, W., Dijksterhuis, A., van Baaren, R. B. (2011a). When Pinocchio acts like a human, a wooden hand becomes embodied. Action co-representation for non-biological agents. Neuropsychologia, 49(5), 1373-1377.
Müller, B. C., Kühn, S., van Baaren, R. B., Dotsch, R., Brass, M., Dijksterhuis, A. (2011b). Perspective taking eliminates differences in co-representation of out-group members’ actions. Experimental Brain Research, 211(3-4), 423-428.
Nuku, P., & Bekkering, H. (2008). Joint attention: Inferring what others perceive (and don’t perceive). Consciousness and Cognition, 17(1), 339-349.
Ondobaka, S., de Lange, F. P., Newman-Norlund, R. D., Wiemers, M., Bekkering, H. (2012). Interplay between action and movement intentions during social interaction. Psychological Science, 23(1), 30–35.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. (2014). R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1-117. Available at http://CRAN.R-project.org/package=nlme.
Posner, M.I., Cohen, Y., (1984). Components of visual orienting. In: Bouma, H., Bouwhuis, D. (Eds.), Attention and Performance, vol. X. Lawrence Erlbaum, London, pp. 531-554.
Prinz, W. (1997). Perception and action planning. European journal of cognitive psychology, 9(2), 129-154.
Prinz, W., Sebanz, N. (2003). Representing others ’ actions : just like one ’ s own ? Cognition, 88, 11-21.
Raftery, A. E. (1995). Bayesian model selection in social research. Sociological Methodology, 25, 111–163.
Risko, E. F., Laidlaw, K., Freeth, M., Foulsham, T., Kingstone, A. (2012). Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience, 6.
Ruys, K. I., Aarts, H. (2010). When competition merges people's behavior: Interdependency activates shared action representations. Journal of Experimental Social Psychology, 46(6), 1130-1133. https://doi.org/10.1016/j.jesp.2010.05.016
Sebanz, N., Knoblich, G., Prinz, W. (2003). Representing others' actions: Just like one's own? Cognition, 88(3), B11-B21.
Sebanz, N., Knoblich, G., Prinz, W., Wascher, E. (2006). Twin peaks: An ERP study of action planning and control in co-acting individuals. Journal of Cognitive Neuroscience, 18(5), 859-870.
Shi, J., Weng, X., He, S., Jiang, Y. (2010). Biological motion cues trigger reflexive attentional orienting. Cognition, 117, 348-354.
Skarratt, P. A., Cole, G. G., Kingstone, A. (2010). Social inhibition of return. Acta Psychol (Amst), 134(1), 48-54. https://doi.org/10.1016/j.actpsy.2009.12.003
Skarratt, P. A., Cole, G. G., Kuhn, G. (2012). Visual cognition during real social interaction. Frontiers in Human Neuroscience, 6, 196.
Smilek, D., Birmingham, E., Cameron, D., Bischof, W., & Kingstone, A. (2006). Cognitive ethology and exploring attention in real-world scenes. Brain Research, 1080(1), 101-119.
Teufel, C., Alexis, D. M., Clayton, N. S., Davis, G. (2010). Mental-state attribution drives rapid, reflexive gaze following. Attention, Perception, & Psychophysics, 72(3), 695-705.
Tsai, C.-C., Brass, M. (2007). Does the human motor system simulate Pinocchio's actions? Co-acting with a human hand versus a wooden hand in a dyadic interaction. Psychological Science, 18(12), 1058-1062.
Tsai, C.-C., Kuo, W.-J., Hung, D. L., Tzeng, O. J. (2008). Action co-representation is tuned to other humans. Journal of Cognitive Neuroscience, 20(11), 2015-2024.
Tsai, C.-C., Sebanz, N., Knoblich, G. (2011). The GROOP effect: Groups mimic group actions. Cognition, 118(1), 135-140.
Vlainic, E., Liepelt, R., Colzato, L. S., Prinz, W., Hommel, B. (2010). The virtual co-actor: the social Simon effect does not rely on online feedback from the other. Frontiers in psychology, 1(2), 208.
Wagenmakers, E. J. (2007). A practical solution to the pervasive problems ofp values. Psychonomic bulletin & review, 14(5), 779-804.
Welsh, T. N. (2009). When 1+ 1= 1: The unification of independent actors revealed through joint Simon effects in crossed and uncrossed effector conditions. Human Movement Science, 28(6), 726-737.
Welsh, T. N., Elliott, D., Anson, J. G., Dhillon, V., Weeks, D. J., Lyons, J. L., & Chua, R. (2005). Does Joe influence Fred’s action?. Neuroscience Letters, 385(2), 99–104.
Welsh, T. N., Higgins, L., Ray, M., Weeks, D. J. (2007). Seeing vs. believing: Is believing sufficient to activate the processes of response co-representation? Human Movement Science, 26(6), 853–866.
Welsh, T. N., Lyons, J., Weeks, D. J., Anson, J. G., Chua, R., Mendoza, J., Elliott, D. (2007). Within- and between-person inhibition of return: Observation is as good as performance. Psychonomic Bulletin and Review, 14, 950–956.
Welsh, T. N., McDougall, L. M., & Weeks, D. J. (2009). The performance and observation of action shape future behaviour. Brain and Cognition, 71(2), 64–71.
Welsh, T. N., Ray, M. C., Weeks, D. J., Dewey, D., & Elliott, D. (2009). Does Joe influence Fred’s action? Not if Fred has autism spectrum disorder. Brain Research, 1248, 141–148
Wiese, E., Wykowska, A., Zwickel, J., & Müller, H. J. (2012). I see what you mean: how attentional selection is shaped by ascribing intentions to others. PloS one, 7(9), e45391.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atkinson, M.A., Millett, A.C., Doneva, S.P. et al. How social is social inhibition of return?. Atten Percept Psychophys 80, 1892–1903 (2018). https://doi.org/10.3758/s13414-018-1546-3
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-018-1546-3