Abstract
Background and objective
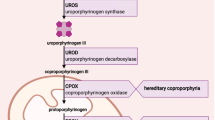
Acute intermittent porphyria is an autosomal dominant disorder caused by deficient activity of the third enzyme in the haem biosynthetic pathway, porphobilinogen deaminase. It is characterised by acute, potentially life-threatening neurological attacks that are precipitated by various drugs, reproductive hormones and other factors. During acute attacks, the porphyrin precursors 5-aminolevulinic acid and porphobilinogen accumulate and are excreted at high concentrations in the urine. Current treatment is based on glucose loading and parenteral haem replenishment, which reduce the accumulation of 5-aminolevulinic acid and porphobilinogen. Recently, a new form of treatment based on porphobilinogen deaminase enzyme replacement therapy has been shown to be effective in an acute intermittent porphyria mouse model which, during phenobarbital (phenobarbitone) induction of haem biosynthesis, mimics the biochemical pattern of acute porphyric attacks. The objective of the present study was to investigate the safety, pharmacokinetics and pharmacodynamics of recombinant human porphobilinogen deaminase (P 9808), administered to healthy subjects and asymptomatic porphobilinogen deaminase-deficient subjects with high concentrations of porphobilinogen, the substrate of porphobilinogen deaminase.
Study design
Forty individuals participated in this two-part study: 20 asymptomatic porphobilinogen deaminase-deficient subjects (both male and female) with ≥4 times the upper reference urinary porphobilinogen level, and 20 healthy male subjects. Four different doses of recombinant human porphobilinogen deaminase were studied (0.5, 1, 2 and 4 mg/kg bodyweight). Part A included 12 asymptomatic porphobilinogen deaminase-deficient subjects, and the enzyme was administered in an open-label, single-dose design. Part B included 20 asymptomatic porphobilinogen deaminase-deficient subjects and 20 healthy subjects. The same enzyme dosages were administered as divided doses every 12 hours for 4 consecutive days in a randomised, double-blinded, placebo-controlled design. The washout period between Parts A and B was 2 weeks.
Methods
The concentrations of recombinant human porphobilinogen deaminase and titres of antibodies against recombinant human porphobilinogen deaminase were analysed by ELISA. Plasma porphobilinogen and 5-aminolevulinic acid concentrations were analysed using a novel liquid chromatography-tandem mass spectrometry method. Urinary porphobilinogen, 5-aminolevulinic acid and porphyrin concentrations, as well as plasma porphyrin concentrations, were analysed using standard methods. The pharmacodynamic effect of the enzyme was studied through changes in plasma porphobilinogen concentrations.
Results
No serious adverse events were observed. Seven subjects (four healthy men and three asymptomatic porphobilinogen deaminase-deficient subjects) developed antibodies against recombinant human porphobilinogen deaminase but did not experience allergic manifestations. The mean elimination half-lives of the highest doses of recombinant human porphobilinogen deaminase ranged between 1.7 and 2.5 hours for both healthy men and asymptomatic porphobilinogen deaminase-deficient subjects. The area under the plasma concentration-time curve was proportional to the respective dose. In asymptomatic porphobilinogen deaminase-deficient subjects, plasma porphobilinogen concentrations decreased below measurable levels almost instantaneously after administration of any dose of the enzyme. The effect lasted for approximately 2 hours, after which the plasma porphobilinogen concentration slowly increased, reaching about 70% of the initial values 12 hours after administration. There was no effect on plasma 5-aminolevulinic acid concentrations, and there was a transitory increment in porphyrin concentrations. The corresponding concentrations of metabolites in the urine reflected the pattern observed in the plasma.
Conclusions
The recombinant human porphobilinogen deaminase enzyme preparation was found to be safe to administer and effective for removal of the accumulated metabolite porphobilinogen from plasma and urine. The pharmacokinetic profile of recombinant human porphobilinogen deaminase showed dose proportionality, and the elimination half-life was about 2.0 hours for the two highest doses. Thus, clinical grounds were established for investigation of the therapeutic efficacy of the enzyme during periods of overt disease in patients with acute intermittent porphyria.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Anderson KE, Sassa S, Bishop DF, et al. Disorders of heme biosynthesis: x-linked sideroblastic anemia and the porphyrias. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill, 2001: 2991–3062
Shoolingin-Jordan PM, Al-Dbass A, McNeill LA, et al. Human porphobilinogen deaminase mutations in the investigation of the mechanism of dipyrromethane cofactor assembly and tetrapyrrole formation. Biochem Soc Trans 2003; 31 (Pt3): 731–5
Thunell S, Harper P, Brock A, et al. Porphyrins, porphyrin metabolism and porphyrias: II. Diagnosis and monitoring in the acute porphyrias. Scand J Clin Lab Invest 2000; 60(7): 541–59
Fraunberg MV, Pischik E, Udd L, et al. Clinical and biochemical characteristics and genotype-phenotype correlation in 143 Finnish and Russian patients with acute intermittent porphyria. Medicine (Baltimore) 2005; 84(1): 35–47
Kauppinen R, Timonen K, Mustajoki P. Treatment of the porphyrias. Ann Med 1994; 26: 31–8
Mustajoki P, Tenhunen R, Pierach C, et al. Heme in the treatment of porphyrias and hematological disorders. Semin Hematol 1989; 26: 1–9
Handschin C, Lin J, Rhee J, et al. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 2005; 122(4): 505–15
Johansson A, Möller C, Fogh J, et al. Biochemical characterization of the porphobilinogen deaminase deficient mice during phenobarbital induction of heme synthesis and the effect of enzyme replacement. Mol Med 2003; 9(9–12): 193–9
Johansson A, Nowak G, Möller C, et al. Adenoviral-mediated expression of porphobilinogen deaminase in liver restores the metabolic defect in a mouse model of acute intermittent porphyria. Mol Ther 2004; 10(2): 337–43
Thunell S. Porphyrins, porphyrin metabolism and porphyrias: I. Update. Scand J Clin Lab Invest 2000; 60(7): 509–40
Shoolingin-Jordan PM, McNeill L. Molecular changes in porphobilinogen deaminase in AIP [abstract]. Physiol Res 2003; 52: 24S
Strand LJ, Felsher BF, Redeker AG, et al. Heme biosynthesis in intermittent acute porphyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A 1970; 67: 1315–20
Lindberg RLP, Porcher C, Grandchamp B, et al. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat Genet 1996; 12: 195–9
Floderus Y, Shoolingin-Jordan P, Harper P. Acute intermittent porphyria in Sweden: molecular, functional and clinical consequences of some new mutations found in the porphobilinogen deaminase gene. Clin Genet 2002; 62(4): 288–97
Davis JR, Andelman SL. Urinary delta-aminolevulinic acid (ALA) levels in lead poisoning: I. A modified method for the rapid determination of urinary delta-aminolevulinic acid using disposable ion-exchange chromatography columns. Arch Environ Health 1967; 15: 53–9
Mauzerall D, Granick S. The occurrence and determination of delta-aminolevulinic acid and porphobilinogen in urine. J Biol Chem 1956; 219: 435–46
Bloom KE, Zaider EF, Morledge LJ, et al. Urinary porphyrin excretion in normal children and adults. Am J Kidney Dis 1991; 18: 483–9
Siersema PD, de Rooij FWM, Edixhoven-Bosdijk A, et al. Heme synthesis in chronic renal failure: the effects of hemodialysis, peritoneal dialysis and erythropoietin treatment. Nephron 1995; 71: 297–302
Lim CK, Peters TJ. Urine and faecal porphyrin profiles by reversed-phase high-performance liquid chromatography in the porphyrias. Clin Chim Acta 1984; 139: 55–63
Scantox A/S. Determination of recombinant human porphobilinogen deaminase in human and in cynomolgus monkey plasma by ELISA: validation of method. Scantox 42051. Lille Skensved: Scantox A/S, 2001
Floderus Y, Sardh E, Moller C, et al. Variations in porphobilinogen and 5-aminolevulinic acid concentrations in plasma and urine from asymptomatic carriers of the acute intermittent porphyria gene with increased porphyrin precursor excretion. Clin Chem 2006; 52(4): 701–7
Acknowledgements
The authors want to acknowledge the nurses at the Stockholm Söder Hospital and the staff at the Porphyria Centre Sweden for expert and excellent technical assistance in accomplishing the patient study and the analysis of porphyrin metabolites, respectively. The authors acknowledge Spadille (now renamed as aCROnordic A/S; Hørsholm, Denmark) for their work with the primary data, and Dr Jens Aas Jansen (Jansen Consulting; Charlottenlund, Denmark) for pharmacokinetic advice. The authors are especially grateful to Prof. Stig Thunell for revising the manuscript. The preparation of the manuscript was partially supported by grants from the Karolinska Institute and from the Department of Internal Medicine at Stockholm Söder Hospital. Lillian Rejkaejer is employed by Zymenex A/S, the manufacturer of Porphozym™. The authors have no other conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sardh, E., Rejkjær, L., Andersson, D.E.H. et al. Safety, Pharmacokinetics and Pharmocodynamics of Recombinant Human Porphobilinogen Deaminase in Healthy Subjects and Asymptomatic Carriers of the Acute Intermittent Porphyria Gene Who Have Increased Porphyrin Precursor Excretion. Clin Pharmacokinet 46, 335–349 (2007). https://doi.org/10.2165/00003088-200746040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746040-00006