Abstract
Background: Achieving the maximum reduction in cardiovascular morbidity and mortality is the primary goal of blood pressure (BP) control. Current guidelines recommend several antihypertensive classes as first-line therapy for this purpose but the decision on which agent/s to use will likely be based upon the treating physician’s clinical experience. Observational studies provide a useful way of ascertaining the efficacy and tolerability of an anti-hypertensive in a real-life clinical setting.
Objective: The aim of this observational study was to determine the efficacy, tolerability and physician/patient satisfaction with long-acting nifedipine (gastrointestinal therapeutic system [GITS]/osmotic-controlled release oral delivery system [OROS]) in a large multinational cohort of hypertensive patients.
Methods: This observational study was conducted in adults (aged ≥18 years) with previously untreated or treated hypertension. The decision to prescribe nifedipine 30 or 60 mg once daily was made by the treating physician. Patients then attended up to three clinic visits any time over a 12-week period when medication could be up- or down-titrated or switched. The mean reduction in systolic BP (SBP)/diastolic BP (DBP) from first visit and whether target BP (<140/<90 mmHg or <130/<80 mmHg [for patients with diabetes mellitus]) had been achieved were recorded at the final visit and stratified according to hypertension grade and presence of cardiovascular risk factors. Subjective assessment of efficacy was reported by physicians and patients. All adverse events and their possible relationship to study drug were recorded. All assessments were performed on patients who received at least one dose of nifedipine GITS/OROS.
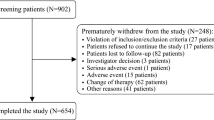
Results: A total of 14 344 patients received nifedipine GITS/OROS treatment (58.7% male; 77.7% non-diabetic; mean age 57.5 years); 14266 had at least one follow-up visit over a mean 10.2-week period, and 8000 patients had three visits over a mean 12-week period. Initially, 12 826 (89.4%) patients received nifedipine 30 mg, and 6912 patients (48.2%) overall received concomitant antihypertensive agents. The overall mean reduction in SBP/DBP was −27.7/−14.1 mmHg; BP reduction was linked to hypertension grade, age, the presence of five or more cardiovascular risk factors, and prior treatment. Target BP was achieved in 2485/7432 patients (33.4%) receiving nifedipine GITS/OROS monotherapy and in 1751/6912 (25.3%) receiving combination therapy (i.e. GITS/OROS plus any other antihypertensive agent). Non-diabetic patients with moderate (n = 3413) and high (n= 1138) risk reached their target BP goal in 62.5% and 54.2% of cases, respectively; the corresponding values in diabetic patients (moderate-added risk n = 8; high-added risk n=684) were 75.0% and 54.8%, respectively. A total of 229 patients (1.6%) reported experiencing 286 adverse events. Physician/patient satisfaction with treatment was high.
Conclusion: Long-acting nifedipine GITS/OROS, alone or in combination with other antihypertensive agents, provides effective and well tolerated treatment of hypertension in a broad spectrum of patients routinely seen in day-to-day clinical practice.









Similar content being viewed by others
References
Lawes CM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21(4): 707–16
Martiniuk AL, Lee CM, Lawes CM, et al. Hypertension: the prevalence and population-attributable fraction for mortality from cardiovascular disease in the Asia-Pacific region. J Hypertens 2007; 25(1): 73–9
Liu LS, Caguioa ES, Park CG, et al. Reducing stroke risk in hypertensive patients: Asian Consensus Conference Recommendations. Int J Stroke 2006; 1(3): 150–7
Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension. J Hypertens 2007; 25(6): 1105–87
Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 2003; 289(19): 2560–72
Huo Y, Zhang J, He Q, et al. Efficacy and safety of nifedipine GITS in Chinese patients with hypertension: a post-marketing surveillance study. Blood Press 2007 Suppl.; 16(1): 18–23
Ueng K-C, Chen Z-C, Yeh P-S, et al. Nifedipine OROS in Chinese patients with hypertension: results of a post-marketing surveillance study in Taiwan. Blood Press 2005; 14Suppl. 1: 32–8
Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000; 356(9227): 366–72
Poole-Wilson PA, Lubsen J, Kirwan B-A, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treating (ACTION trial): randomised, controlled trial. Lancet 2004; 364(9437): 849–57
Saito I, Saruta T, ADVANCE-Combi Study Group. Controlled release nifedipine and valsartan combination therapy in patients with essential hypertension: the nifedipine CR and valsartan cost-effectiveness combination (ADVANCE-Combi) study. Hypertens Res 2006; 29(10): 789–96
Hasebe N, Kikuchi K. Combined-release nifedipine and candesartan low-dose combination therapy in patients with essential hypertension: the NICE Combi (Nifedipine and Candesartan Combination) Study. J Hypertens 2005; 23(2): 445–53
Snider ME, Nuzum DS, Veverka A. Long-acting nifedipine in the management of the hypertensive patient. Vasc Health Risk Manag 2008; 4(6): 1249–57
Lundy A, Lufti N, Beckey C. Review of nifedipine GITS in the treatment of high risk patients with coronary artery disease and hypertension. Vasc Health Risk Manag 2009; 5(1): 429–40
Grundy JS, Foster RT. The nifedipine gastrointestinal therapeutic system (GITS): evaluation of pharmaceutical, pharmacokinetic and pharmacological properties. Clin Pharmacokinet 1996; 30(1): 28–51
Heagerty AM. Nifedipine gastrointestinal therapeutic system: hypertension management to improve cardiovascular outcomes. Int J Clin Pract 2005; 59(9): 1112–9
Mohiuddin SM, Hilleman DE. Substituting nifedipine-GITS for immediate-release calcium-channel antagonists in patients with stable angina pectoris. Curr Ther Res 1991; 50: 546–52
Myers MG, Toal CB. Comparative efficacy of two long-acting formulations of nifedipine in the treatment of hypertension. The Switch Study Investigators. Can J Cardiol 1995; 11: 913–7
European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–53
1999 World Health Organization — International Society of Hypertension Guidelines for the management of hypertension. Guidelines subcommittee. J Hypertens 1999; 17: 151–83
Ogden LG, He J, Lydick E, et al. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension 2000; 35(2): 539–43
Qin X, Jackson R, Marshall R, et al. Modelling the potential impact of population-wide and targeted high-risk blood pressure-lowering strategies on cardiovascular disease in China. Eur J Cardiovasc Prev Rehabil 2009; 16(1): 96–101
Runlin G, Junren Z, Guozhang L, et al. Efficacy and safety of nifedipine GITS in Asians with hypertension: results of a post-marketing surveillance study in China. Clin Drug Invest 2007; 27(8): 565–72
Bakris G, Hill M, Mancia G, et al. Achieving blood pressure goals globally: five core actions for health-care professionals. A worldwide call to action. J Hum Hypertens 2008; 22(1): 63–70
Kirby BJ, Kitchin NR. A comparison of the effects of two modified release preparations of nifedipine-nifedipine retard 10 mg twice daily and nifedipine GITS 20 mg once daily: in the treatment of mild to moderate hypertension. Int J Clin Pract 1999; 53(5): 339–43
Croom KF, Wellington K. Modified-release nifedipine: a review of the use of modified-release formulations in the treatment of hypertension and angina pectoris. Drugs 2006; 66(4): 497–528
Meredith PA, Elliott HL. Dihydropyridine calcium channel blockers: basic pharmacological similarities but fundamental therapeutic differences. J Hypertens 2004; 22(9): 1641–8
Brown MJ, Toal CB. Formulation of long-acting nifedipine tablets influences the heart rate and sympathetic nervous system response in hypertensive patients. Br J Clin Pharmacol 2008; 65(5): 646–52
Acknowledgements
This study and data analyses were funded by Bayer Schering Pharma, Leverkusen, Germany. The Bayer Global NIS department was responsible for global project management. The Contract Research Organisation (CRO) Institute Dr Schauerte, Oberhaching, Germany, was responsible for study logistics, data monitoring, data capture, data management of cases with adverse events or suspected adverse events, statistical analysis and reporting of study results. PAREXEL MMS provided writing assistance. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueng, KC., Ningling, S., Maksod, A.E. et al. Efficacy and Tolerability of Long-Acting Nifedipine GITS/OROS Monotherapy or Combination Therapy in Hypertensive Patients. Clin. Drug Investig. 31, 631–642 (2011). https://doi.org/10.2165/11588970-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11588970-000000000-00000