Abstract
Background
Although tumor regression and nodal status are reported to be useful prognostic factors for patients with oesophageal cancer who are treated with neoadjuvant chemoradiotherapy, the clinical effects of those factors remain to be explained fully in neoadjuvant chemotherapy. Additionally, factor predictive of systemic disease after neoadjuvant therapy remain unexplored.
Methods
The impact of pathological tumor regression and the number of involved lymph nodes on survival and the occurrence of systemic disease were examined in 405 patients with esophageal squamous cell carcinoma who received neoadjuvant chemotherapy,
Results
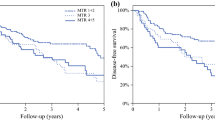
Among the 405 patients studied, 96 (23.7%) achieved good response, whereas 309 (76.3%) were classified as poor response to neoadjuvant chemotherapy. Systemic disease occurred in 136 patients (34.6%) of 393 patients who underwent curative esophagectomy. The number of involved lymph nodes and pathological tumor regression were associated with survival and the occurrence of systemic disease. Multivariate analysis showed that the number of involved lymph nodes was identified as an independent factor associated with both survival and the occurrence of systemic disease, together with the latest AJCC ypstage. However, tumor regression was not found to be an independent factor associated with survival and systemic disease in multivariate analysis.
Conclusions
Posttreatment nodal status rather than pathological tumor regression seems to be useful for predicting prognosis and the occurrence of systemic disease in patients with esophageal squamous cell carcinoma who underwent neoadjuvant chemotherapy. Additional systemic therapy may be needed in patients with several involved lymph nodes remaining after neoadjuvant therapy.


Similar content being viewed by others
References
Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92.
Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–34.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–33.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Miyata H, Yamasaki M, Miyazaki Y, et al. Clinical importance of supraclavicular lymph node metastasis after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. Ann Surg. 2015;262:280–5.
Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Bekkar S, Gronnier C, Renaud F, et al. Multicentre study of neoadjuvant chemotherapy for stage I and II oesophageal cancer. Br J Surg. 2016;103:855–62.
Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354–60.
Klevebro F, Alexandersson von Döbeln G, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27:660–7.
Hoeppner J, Lordick F, Brunner T, et al. Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer. 2016;16:503.
Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43:752–5.
Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005; 23: 4330–6.
Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005; 103: 1347–55.
Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer. Implication for response classification. Ann Surg. 2005;5:684–92.
Reynolds JV, Muldoon C, Hollywood D, et al. Long-term outcomes following neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg. 2007;245:707–16.
Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–71.
Koen Talsma A, Shapiro J, Looman CW, et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: prognostic and therapeutic impact on survival. Ann Surg. 2014;260:786–92.
Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8.
Kelsen DP, Winter KA, Gunderson LL, et al. A random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007 20;25:3719–25.
Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385–91.
Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–22.
Miyata H, Yamasaki M, Takahashi T, et al. Relevance of 18F-fluorodeoxyglucose positron emission tomography-positive lymph nodes after neoadjuvant chemotherapy for squamous cell oesophageal cancer. Br J Surg 2013;100:1490–7.
Miyata H, Yano M, Doki Y, et al. A prospective trial for avoiding cervical lymph node dissection for thoracic esophageal cancers, based on intra-operative genetic diagnosis of micrometastasis in recurrent laryngeal nerve chain nodes. J Surg Oncol. 2006;93:477–84.
Japanese Society for Esophageal Diseases: Guidelines for the clinical and pathologic studies on carcinoma of the esophagus (ed 10). Tokyo, Japan, Kanehara Syuppan, 2007.
Miyata H, Yamasaki M, Takiguchi S, et al. Prognostic value of endoscopic biopsy findings after induction chemoradiotherapy with and without surgery for esophageal cancer. Ann Surg. 2011;253:279–84.
Brierley J, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours eighth edition. Oxford: Wiley, 2017.
Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual, 8th edn. New York: Springer, 2017.
Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56.
Smyth EC, Fassan M, Cunningham D, et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34:2721–7.
Schmidt T, Sicic L, Blank S, et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer. 2014;110:1712–20.
Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathological correlations. Cancer 1994;73:2680–6.
Donohoe CL, O’Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg. 2013;258:784–92.
Robb WB, Dahan L, Mornex F, et al. Impact of neoadjuvant chemoradiation on lymph node status in esophageal cancer: post hoc analysis of a randomized controlled trial. Ann Surg. 2015;261:902–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyata, H., Tanaka, K., Makino, T. et al. The Impact of Pathological Tumor Regression and Nodal Status on Survival and Systemic Disease in Patients Undergoing Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 25, 2409–2417 (2018). https://doi.org/10.1245/s10434-018-6507-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6507-5