Abstract
Background
Malignant pleural mesothelioma (MPM), a devastating neoplasm, is traditionally considered to be resistant to antitumor therapy. Identification of clinical prognostic indicators is therefore needed. Although the C-reactive protein/albumin ratio (CAR) has been used to predict the prognosis of many types of malignancy, its utility in patients with MPM is unknown.
Methods
The data of 100 patients diagnosed as having MPM from 1995 to 2015 at the National Kyushu Cancer Center and Kyushu University were analyzed. The CAR was calculated as serum C-reactive protein concentration divided by albumin concentration. A cutoff for CAR was set at 0.58 according to a receiver operating characteristics curve for 1-year survival.
Results
Thirty-five of the 100 (35.0%) patients were classified as having a high CAR. A high CAR was significantly associated with advanced clinical stage (p < 0.001) and chemotherapy alone (p = 0.002). Patients with a high CAR had significantly shorter overall survival (OS) (p < 0.001) and disease- or progression-free survival (DFS/PFS) (p < 0.001). These associations between CAR and prognosis remained significant after propensity score-matching. In multivariate analysis, a high CAR was an independent predictor of shorter OS and DFS/PFS (p = 0.003 and p = 0.008, respectively). Multivariate analyses of the subgroups of patients who had received chemotherapy and of patients who had undergone surgery also showed that a high CAR was an independent predictor of shorter OS and DFS/PFS.
Conclusions
CAR is an independent predictor of prognosis in MPM patients. This prognostic index contributes to clinicians’ ability to predict benefit from treatment. Further larger, prospective studies are necessary to validate these findings.


Similar content being viewed by others
References
Ladanyi M, Zauderer MG, Krug LM, et al. New strategies in pleural mesothelioma: BAP1 and NF2 as novel targets for therapeutic development and risk assessment. Clin Cancer Res. 2012;18(17):4485–90.
Andujar P, Lacourt A, Brochard P, Pairon JC, Jaurand MC, Jean D. Five years update on relationships between malignant pleural mesothelioma and exposure to asbestos and other elongated mineral particles. J Toxicol Environ Health B. 2016;19(5–6):151–72.
Perrot M, Wu L, Wu M, Cho BCJ. Radiotherapy for the treatment of malignant pleural mesothelioma. Lancet Oncol. 2017;18(9):e532–e42.
Bronte G, Incorvaia L, Rizzo S, et al. The resistance related to targeted therapy in malignant pleural mesothelioma: why has not the target been hit yet? Crit Rev Oncol Hematol. 2016;107:20–32.
Haas AR, Sterman DH. Malignant pleural mesothelioma: update on treatment options with a focus on novel therapies. Clin Chest Med. 2013;34(1):99–111.
Ceresoli GL, Locati LD, Ferreri AJ, et al. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer. 2001;34(2):279–87.
Shoji F, Morodomi Y, Akamine T, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer. 2016;98:15–21.
Akamine T, Toyokawa G, Matsubara T, et al. Significance of the preoperative CONUT score in predicting postoperative disease-free and overall survival in patients with lung adenocarcinoma with obstructive lung disease. Anticancer Res. 2017;37(5):2735–42.
Nakashima Y, Saeki H, Nakanishi R, et al. (2017) Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000002252.
Shoji F, Haratake N, Akamine T, et al. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37(2):741–47.
Toyokawa G, Kozuma Y, Matsubara T, et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. 2017;9(9):2942–51.
Takamori S, Toyokawa G, Taguchi K, et al. The controlling nutritional status score is a significant independent predictor of poor prognosis in patients with malignant pleural mesothelioma. Clin Lung Cancer. 2017;18(4):e303–e13.
Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–96.
Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, Sharma R. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol. 2012;7(3):587–94.
Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16(23):5805–13.
Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med. 2009;9(1):30–33.
Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PloS One. 2013;8(3):e59321.
Ide S, Toiyama Y, Okugawa Y, et al. Clinical significance of C-reactive protein-to-albumin ratio with rectal cancer patient undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2017;37(10):5797–804.
Otowa Y, Nakamura T, Yamamoto M, et al. C-reactive protein to albumin ratio is a prognostic factor for patients with cStage II/III esophageal squamous cell cancer. Dis Esophagus. 2017;30(12):1–5.
Arima K, Yamashita YI, Hashimoto D, et al. (2017) Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg. https://doi.org/10.1016/j.amjsurg.2017.08.016.
Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep. 2017;7(1):2993.
Koh YW, Lee HW. Prognostic impact of C-reactive protein/albumin ratio on the overall survival of patients with advanced nonsmall cell lung cancers receiving palliative chemotherapy. Medicine. 2017;96(19):e6848.
Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285.
Galateau-Salle F, Churg A, Roggli V, Travis WD. The 2015 World Health Organization classification of tumors of the pleura: advances since the 2004 classification. J Thorac Oncol. 2016;11(2):142–54.
Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36(1):76–81.
Prentice RL, Marek P. A qualitative discrepancy between censored data rank tests. Biometrics. 1979;35(4):861–67.
Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104–11.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54.
Kemik O, Sumer A, Kemik AS, et al. The relationship among acute-phase response proteins, cytokines and hormones in cachectic patients with colon cancer. World J Surg Oncol. 2010;8:85.
Ilhan N, Ilhan N, Ilhan Y, Akbulut H, Kucuksu M. C-reactive protein, procalcitonin, interleukin-6, vascular endothelial growth factor and oxidative metabolites in diagnosis of infection and staging in patients with gastric cancer. World J Gastroenterol. 2004;10(8):1115–20.
Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13.
Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002.
Kadariya Y, Menges CW, Talarchek J, et al. Inflammation-Related IL1beta/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev Res. 2016;9(5):406–14.
Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117–23.
Yao ZH, Tian GY, Yang SX, et al. Serum albumin as a significant prognostic factor in patients with malignant pleural mesothelioma. Tumour Biol Oncodev Biol Med. 2014;35(7):6839–45.
Linton A, van Zandwijk N, Reid G, Clarke S, Cao C, Kao S. Inflammation in malignant mesothelioma—friend or foe? Ann Cardiothorac Surg. 2012;1(4):516–22.
Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16(1):145–52.
Bille A, Krug LM, Woo KM, Rusch VW, Zauderer MG. Contemporary analysis of prognostic factors in patients with unresectable malignant pleural mesothelioma. J Thorac Oncol. 2016;11(2):249–55.
Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1996;111(4):815-25; discussion 25–6.
Ceresoli GL, Grosso F, Zucali PA, et al. Prognostic factors in elderly patients with malignant pleural mesothelioma: results of a multicenter survey. Br J Cancer. 2014;111(2):220–26.
Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117(1):54–63; discussion 63-5.
Schouwink JH, Kool LS, Rutgers EJ, et al. The value of chest computer tomography and cervical mediastinoscopy in the preoperative assessment of patients with malignant pleural mesothelioma. Ann Thorac Surg. 2003;75(6):1715–8; discussion 18–9.
Heelan RT, Rusch VW, Begg CB, Panicek DM, Caravelli JF, Eisen C. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol. 1999;172(4):1039–47.
Yamagishi T, Fujimoto N, Nishi H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer. 2015;90(1):111–17.
Tagawa T, Anraku M, Morodomi Y, et al. Clinical role of a new prognostic score using platelet-to-lymphocyte ratio in patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy. J Thorac Dis. 2015;7(11):1898–906.
Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol. 2011;6(11):1923–29.
Acknowledgment
The authors thank Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Disclosure
The authors declare no conflicts of interest in association with the present study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2018_6385_MOESM1_ESM.tiff
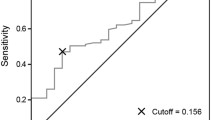
Supplementary Fig. 1 Receiver operating characteristic curve for 1-year survival showing that C-reactive protein/albumin ratio has a good diagnostic ability with 80.0% sensitivity and 67.9% specificity. The area under the curve was calculated as 0.782. Supplementary material 1 (TIFF 2787 kb)
10434_2018_6385_MOESM2_ESM.tiff
Supplementary Fig. 2 Kaplan–Meier curves of overall survival (OS) and disease- or progression-free survival (DFS/PFS) according to C-reactive protein/albumin ratio in patients with stage I/II disease. The (a) OS and (b) DFS/PFS of the groups did not differ to a statistically extent (p = 0.079 and p = 0.062, respectively). Supplementary material 2 (TIFF 2787 kb)
Rights and permissions
About this article
Cite this article
Takamori, S., Toyokawa, G., Shimokawa, M. et al. The C-Reactive Protein/Albumin Ratio is a Novel Significant Prognostic Factor in Patients with Malignant Pleural Mesothelioma: A Retrospective Multi-institutional Study. Ann Surg Oncol 25, 1555–1563 (2018). https://doi.org/10.1245/s10434-018-6385-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6385-x