Abstract
Background
Despite randomized trials addressing adjuvant therapy (AT) for pancreas cancer, the ideal time to initiate therapy remains undefined. Retrospective analyses of the ESPAC-3 trial demonstrated that time to initiation of AT did not impact overall survival (OS). Given the absence of confirmatory data outside of a clinical trial, we sought to determine if AT timing in routine clinical practice is associated with OS differences.
Methods
Perioperative data of pancreatectomies for ductal adenocarcinoma from five institutions (2005–2015) were assessed. Delay in AT was defined as initiation >12 weeks after surgery. Multivariate analysis was performed to identify predictors of mortality.
Results
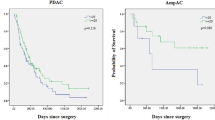
Of 867 patients, 172 (19.8%) experienced omission of AT. Improved OS was observed in patients who received AT compared with patients who did not (24.8 vs. 19.1 months, p < 0.01). Information on time to initiation of AT was available in 488 patients, of whom 407 (83.4%) and 81 (16.6%) received chemotherapy ≤12 and >12 weeks after surgery, respectively. There were no differences in recurrence-free survival or OS (all p > 0.05) between the timely and delayed AT groups. After controlling for perioperative characteristics and tumor pathology, patients who initiated AT ≤ 12 or > 12 weeks after surgery had a 50% lower odds of mortality than patients who only underwent resection (p < 0.01).
Conclusions
In a multi-institutional experience of resected pancreas cancer, delayed initiation of AT was not associated with poorer survival. Patients who do not receive AT within 12 weeks after surgery are still appropriate candidates for multimodal therapy and its associated survival benefit.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–79.
Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220(4):530–6.
Xia BT, Ahmad SA. Clinical Considerations for Pancreatic Cancer. Semin Roentgenol. 2016;51(2):74–81.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10.
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–81.
Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32(6):504–12.
Saeed H, Hnoosh D, Huang B, et al. Defining the optimal timing of adjuvant therapy for resected pancreatic adenocarcinoma: a statewide cancer registry analysis. J Surg Oncol. 2016;114(4):451–55.
Mirkin KA, Greenleaf EK, Hollenbeak CS, Wong J. Time to the initiation of adjuvant chemotherapy does not impact survival in patients with resected pancreatic cancer. Cancer. 2016;122(19):2979–87.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260(2):372–77.
Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–42.
Porschen R, Bermann A, Loffler T, et al. Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA-01. J Clin Oncol. 2001;19(6):1787–94.
Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696–704.
Arkenau HT, Bermann A, Rettig K, Strohmeyer G, Porschen R, Arbeitsgemeinschaft Gastrointestinale O. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol. 2003;14(3):395–9.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24.
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Brent T. Xia, Syed A. Ahmad, Ali H. Al Humaidi, Dennis J. Hanseman, Cecilia G. Ethun, Shishir K. Maithel, David A. Kooby, Ahmed Salem, Clifford S. Cho, Sharon M. Weber, Susan J. Stocker, Mark S. Talamonti, David J. Bentrem, and Daniel E. Abbott have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, B.T., Ahmad, S.A., Al Humaidi, A.H. et al. Time to Initiation of Adjuvant Chemotherapy in Pancreas Cancer: A Multi-Institutional Experience. Ann Surg Oncol 24, 2770–2776 (2017). https://doi.org/10.1245/s10434-017-5918-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5918-z