Abstract
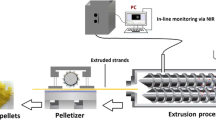
The aims of this study were to prepare hydrogenated soybean phosphatidylcholine (HSPC) matrices by hot melt extrusion and to evaluate resulting matrix potential to extend drug release in regard to drug loading and solubility for oral drug delivery of water-soluble drugs. The liquid crystalline nature of HSPC powder allowed its extrusion at 120°C, which was below its capillary melting point. Model drugs with a wide range of water solubilities (8, 20 and 240 mg/mL) and melting temperatures (160–270°C) were used. Extrudates with up to 70% drug loading were prepared at temperatures below the drugs’ melting points. The original crystalline state of the drugs remained unchanged through the process as confirmed by XRPD and hot-stage microscopy. The time to achieve 80% release (t80) from extrudates with 50% drug loading was 3, 8 and 18 h for diprophylline, caffeine and theophylline, respectively. The effect of matrix preparation method (extrusion vs. compression) on drug release was evaluated. For non-eroding formulations, the drug release retarding properties of the HSPC matrix were mostly not influenced by the preparation method. However, with increasing drug loadings, compressed tablets eroded significantly more than extruded matrices, resulting in 2 to 11 times faster drug release. There were no signs of erosion observed in extrudates with different drugs up to 70% loadings. The mechanical robustness of HSPC extrudates was attributed to the formation of a skin-core structure and was identified as the main reason for the drug release controlling potential of the HSPC matrices produced by hot melt extrusion.









Similar content being viewed by others
References
Grund J, Koerber M, Walther M, Bodmeier R. The effect of polymer properties on direct compression and drug release from water-insoluble controlled release matrix tablets. Int J Pharm. 2014;469(1):94–101.
Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154(1):2–19.
Roberts M, Pulcini L, Mostafa S, Cuppok-Rosiaux Y, Marchaud D. Preparation and characterization of Compritol 888 ATO matrix tablets for the sustained release of diclofenac sodium. Pharm Dev Technol. 2015;20(4):507–12.
Kolbina M, Bodmeier R, Körber M. Saturated phosphatidylcholine as matrix former for oral extended release dosage forms. Eur J Pharm Sci. 2017;108:86–92.
van Hoogevest P. Review—an update on the use of oral phospholipid excipients. Eur J Pharm Sci. 2017;108:1–12.
Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004;269(2):509–22.
Grehan L, Killion JA, Devine DM, Kenny EK, Devery S, Higginbotham CL, et al. The development of hot melt extruded biocompatible controlled release drug delivery devices. Int J Polym Mater Polym Biomater. 2014;63(9):476–85.
Kipping T, Rein H. Continuous production of controlled release dosage forms based on hot-melt extruded gum arabic: formulation development, in vitro characterization and evaluation of potential application fields. Int J Pharm. 2016;497(1–2):36–53.
Kallakunta VR, Tiwari R, Sarabu S, Bandari S, Repka MA. Effect of formulation and process variables on lipid based sustained release tablets via continuous twin screw granulation: a comparative study. Eur J Pharm Sci. 2018;121:126–38.
Siepmann F, Muschert S, Flament MP, Leterme P, Gayot A, Siepmann J. Controlled drug release from Gelucire-based matrix pellets: experiment and theory. Int J Pharm. 2006;317(2):136–43.
Güres S, Siepmann F, Siepmann J, Kleinebudde P. Drug release from extruded solid lipid matrices: theoretical predictions and independent experiments. Eur J Pharm Biopharm. 2012;80(1):122–9.
Sato H, Miyagawa Y, Okabe T, Miyajima M, Sunada H. Dissolution mechanism of diclofenac sodium from wax matrix granules. J Pharm Sci. 1997;86(8):929–34.
De Brabander C, Vervaet C, Fiermans L, Remon JP. Matrix mini-tablets based on starch/microcrystalline wax mixtures. Int J Pharm. 2000;199(2):195–203.
Liu J, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2001;52(2):181–90.
Roblegg E, Jäger E, Hodzic A, Koscher G, Mohr S, Zimmer A, et al. Development of sustained-release lipophilic calcium stearate pellets via hot melt extrusion. Eur J Pharm Biopharm. 2011;79(3):635–45.
Laukamp EJ, Vynckier A-K, Voorspoels J, Thommes M, Breitkreutz J. Development of sustained and dual drug release co-extrusion formulations for individual dosing. Eur J Pharm Biopharm. 2015;89:357–64.
Hasa D, Perissutti B, Grassi M, Zacchigna M, Pagotto M, Lenaz D, et al. Melt extruded helical waxy matrices as a new sustained drug delivery system. Eur J Pharm Biopharm. 2011;79(3):592–600.
Vithani K, Cuppok Y, Mostafa S, Slipper IJ, Snowden MJ, Douroumis D. Diclofenac sodium sustained release hot melt extruded lipid matrices. Pharm Dev Technol. 2014;19(5):531–8.
Monteyne T, Adriaensens P, Brouckaert D, Remon J-P, Vervaet C, De Beer T. Stearic acid and high molecular weight PEO as matrix for the highly water soluble metoprolol tartrate in continuous twin-screw melt granulation. Int J. 2016;512(1):158–67.
Vaingankar P, Amin P. Continuous melt granulation to develop high drug loaded sustained release tablet of metformin HCl. Asian J Pharm Sci. 2017;12(1):37–50.
Nart V, Beringhs AO, França MT, de Espíndola B, Pezzini BR, Stulzer HK. Carnauba wax as a promising excipient in melt granulation targeting the preparation of mini-tablets for sustained release of highly soluble drugs. Mater Sci Eng C. 2017;70:250–7.
Chapman D, Williams RM, Ladbrooke BD. Physical studies of phospholipids. VI. Thermotropic and lyotropic mesomorphism of some 1,2-diacyl-phosphatidylcholines (lecithins). Chem Phys Lipids. 1967;1(5):445–75.
Ghalanbor Z, Körber M, Bodmeier R. Improved lysozyme stability and release properties of poly (lactide-co-glycolide) implants prepared by hot-melt extrusion. Pharm Res. 2010;27(2):371–9.
Shah VP, Tsong Y, Sathe P, Liu J. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15(6):889–96.
Koynova R, Caffrey M. Phases and phase transitions of the phosphatidylcholines. Biochim Biophys Acta Rev Biomembr. 1998;1376(1):91–145.
Chapman D. Liquid crystalline nature of phospholipids. In: Porter RS, Johnson JF, editors. Ordered Fluids and Liquid Crystals: American Chemical Society; 1967. p. 157–66.
Williams RM, Chapman D. Phospholipids, liquids crystals and cell membranes. Prog Chem Fats Other Lipids. 1971;11:1–79.
Byrne P, Chapman D. Liquid crystalline nature of phospholipids. Nature. 1964;202(4936):987–8.
Ladbrooke BD, Chapman D. Thermal analysis of lipids, proteins and biological membranes a review and summary of some recent studies. Chem Phys Lipids. 1969;3(4):304–56.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12.
Bravo-Osuna I, Ferrero C, Jiménez-Castellanos MR. Influence of moisture content on the mechanical properties of methyl methacrylate–starch copolymers. Eur J Pharm Biopharm. 2007;66(1):63–72.
Massing U, Bauer-Brandl A. Tablet containing hydrogenated phospholipids. EP1952805A1, 2008.
Luzzati V, Gulik-Krzywicki T, Tardieu A. Polymorphism of lecithins. Nature. 1968;218(5146):1031–4.
Vergnaud JM. Controlled drug release of oral dosage forms. Boca Raton: CRC Press; 1993.
Guse C, Koennings S, Kreye F, Siepmann F, Goepferich A, Siepmann J. Drug release from lipid-based implants: elucidation of the underlying mass transport mechanisms. Int J Pharm. 2006;314(2):137–44.
Siepmann J, Siepmann F. Mathematical modeling of drug release from lipid dosage forms. Int J Pharm. 2011;418(1):42–53.
Grund J, Körber M, Bodmeier R. Predictability of drug release from water-insoluble polymeric matrix tablets. Eur J Pharm Biopharm. 2013;85(3 Pt A):650–5.
Yang L, Fassihi R. Examination of drug solubility, polymer types, hydrodynamics and loading dose on drug release behavior from a triple-layer asymmetric configuration delivery system. Int J Pharm. 1997;155(2):219–29.
Harland RS, Gazzaniga A, Sangalli ME, Colombo P, Peppas NA. Drug/polymer matrix swelling and dissolution. Pharm Res. 1988;05(8):488–94.
Streubel A, Siepmann J, Dashevsky A, Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J Control Release. 2000;67(1):101–10.
Ide Y, Ophir Z. Orientation development in thermotropic liquid crystal polymers. Polym Eng Sci. 1983;23(5):261–5.
Weiss RA, Huh W, Nicolais L. Novel reinforced polymers based on blends of polystyrene and a thermotropic liquid crystalline polymer. Polym Eng Sci. 1987;27(9):684–91.
Acknowledgements
This work was supported by Phospholipid Research Center (Heidelberg, Germany). The authors thank Eva Hepke (Technische Universität Berlin) for technical support with XRPD measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sanyog Jain
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 3231 kb)
Rights and permissions
About this article
Cite this article
Kolbina, M., Schulte, A., van Hoogevest, P. et al. Evaluation of Hydrogenated Soybean Phosphatidylcholine Matrices Prepared by Hot Melt Extrusion for Oral Controlled Delivery of Water-Soluble Drugs. AAPS PharmSciTech 20, 159 (2019). https://doi.org/10.1208/s12249-019-1366-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1366-3