Abstract
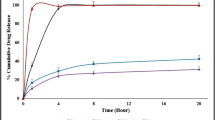
The purpose of this study was to prepare sustained release (SR) matrix tablets using a direct compression incorporated with a post-heating process. Allopurinol was selected due to the water-soluble property and Compritol 888 ATO® (also known as glyceryl behenate) was used as an SR matrix-forming agent. The API, SR material, microcrystalline cellulose, and magnesium stearate (lubricant) were mixed and prepared into a tablet by a direct compression method. The compressed tablets were stored in a dry oven at four temperatures (60, 70, 80, and 90°C) and for three time periods (15, 30, 45 min). The DSC and PXRD data indicated that the crystallinity of the API was not altered by the post-heating method. However, SEM images demonstrated that Compritol 888 ATO® was melted by the post-heating method, and that the melted Compritol 888 ATO® could form a strong matrix. This strong matrix led to the significant sustained release behavior of hydrophilic APIs. As little as 3 mg of Compritol 888 ATO® (0.65% of total tablet weight), when heated at 80°C for 15 min, showed sustained release over 10 h. The post-heating method exerted a significant influence on lipid-based matrix tablets and allowed a reduction in the amount of material required for a water-soluble drug. This will also provide a valuable insight into lipid-based SR tablets and will allow their application to higher quality products and easier processing procedures.









Similar content being viewed by others
References
Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technolo Today. 2000;3:198–204.
Jiménez-Martínez I, Quirino-Barreda T, Villafuerte-Robles L. Sustained delivery of captopril from floating matrix tablets. Int J Pharm. 2008;362:37–43.
Viridén A, Abrahmsén-Alami S, Wittgren B, Larsson A. Release of theophylline and carbamazepine from matrix tablets—consequences of HPMC chemical heterogeneity. Eur J Pharm Biopharm. 2011;78:470–9.
Li L, Wang L, Shao Y, Tian Y, Li C, Li Y, et al. Elucidation of release characteristics of highly soluble drug trimetazidine hydrochloride from chitosan–carrageenan matrix tablets. J Pharm Sci. 2013;102:2644–54.
Peppas N, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46.
Barthelemy P, Laforet J, Farah N, Joachim J. Compritol® 888 ATO: an innovative hot-melt coating agent for prolonged-release drug formulations. Eur J Pharm Biopharm. 1999;47:87–90.
Jeong KH, Woo HS, Kim CJ, Lee KH, Jeon JY, Lee SY, et al. Formulation of a modified-release pregabalin tablet using hot-melt coating with glyceryl behenate. Int J Pharm. 2015;495:1–8.
Rosiaux Y, Jannin V, Hughes S, Marchaud D. Solid lipid excipients—matrix agents for sustained drug delivery. J Control Release. 2014;188:18–30.
Saraiya D, Bolton S. The use of Precirol® to prepare sustained release tablets of theophylline and quinidine gluconate. Drug Dev Ind Pharm. 1990;16:1963–9.
Özyazıcı M, Gökçe EH, Ertan G. Release and diffusional modeling of metronidazole lipid matrices. Eur J Pharm Biopharm. 2006;63:331–9.
Anbalagan P, Liew CV, Heng PWS. Role of dwell on compact deformation during tableting: an overview. J Pharm Investig. 2017;47:173–81.
Li F-Q, Hu J-H, Deng J-X, Su H, Xu S, Liu J-Y. In vitro controlled release of sodium ferulate from Compritol 888 ATO-based matrix tablets. Int J Pharm. 2006;324:152–7.
Schroeder H, Dakkuri A, Deluca P. Sustained release from inert wax matrixes I: drug–wax combinations. J Pharm Sci. 1978;67:350–3.
Smith, A., Datta, S.P., Smith, G.H., Campbell, P.N., Bentley, R., McKenzie, H. Oxford dictionary of biochemistry and molecular biology, 2nd ed. Oxford: Oxford University Press; 2000.
Weiner, A. Lipid excipient for nasal delivery and topical application. US5200393A; 1993.
Thapa RK, Choi H-G, Kim JO, Yong CS. Analysis and optimization of drug solubility to improve pharmacokinetics. J Pharm Investig. 2017;47:95–110.
Keen JM, Foley CJ, Hughey JR, Bennett RC, Jannin V, Rosiaux Y, et al. Continuous twin screw melt granulation of glyceryl behenate: development of controlled release tramadol hydrochloride tablets for improved safety. Int J Pharm. 2015;487:72–80.
Hwang I, Kang C-Y, Park J-B. Advances in hot-melt extrusion technology toward pharmaceutical objectives. J Pharm Investig. 2017;47:123–32.
Obaidat AA, Obaidat RM. Controlled release of tramadol hydrochloride from matrices prepared using glyceryl behenate. Eur J Pharm Biopharm. 2001;52:231–5.
Lee Y, Kim K, Kim M, Choi DH, Jeong SH. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J Pharm Investig. 2017;47:183–01.
Rao M, Ranpise A, Borate S, Thanki K. Mechanistic evaluation of the effect of sintering on Compritol® 888 ATO matrices. AAPS PharmSciTech. 2009;10:355–60.
Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58:265–78.
Food and Drug Administration, Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. Food and Drug Administration, Rockville, MD. 2000.
Emmerson BT. The management of gout. N Engl J Med. 1996;334:445–51.
Roberts M, Pulcini L, Mostafa S, Cuppok-Rosiaux Y, Marchaud D. Preparation and characterization of Compritol 888 ATO matrix tablets for the sustained release of diclofenac sodium. Pharm Dev Technol. 2015;20(4):507–12.
Brubach J, Jannin V, Mahler B, Bourgaux C, Lessieur P, Roy P, et al. Structural and thermal characterization of glyceryl behenate by X-ray diffraction coupled to differential calorimetry and infrared spectroscopy. Int J Pharm. 2007;336:248–56.
Krajacic A, Tucker IG. Matrix formation in sustained release tablets: possible mechanism of dose dumping. Int J Pharm. 2003;251:67–78.
Omelczuk MO, McGinity JW. The influence of thermal treatment on the physical–mechanical and dissolution properties of tablets containing poly (DL-lactic acid). Pharm Res. 1993;10:542–8.
Patere SN, Desai NS, Jain AS, Kadam PP, Thatte UM, Gogtay N, et al. Compritol® 888 ATO a lipid excipient for sustained release of highly water soluble active: formulation, scale-up and IVIVC study. Curr Drug Deliv. 2013;10:548–56.
El-Wahed MA, Refat M, El-Megharbel S. Metal complexes of antiuralethic drug: synthesis, spectroscopic characterization and thermal study on allopurinol complexes. J Mol Struct. 2008;888:416–29.
Kaur A, Goindi S, Katare OP. Thermal analysis and quantitative characterization of compatibility between diflunisal and lipid excipients as raw materials for development of solid lipid nanoparticles. Thermochim Acta. 2016;643:23–32.
Obaidat, R., Alnaief, M., and Mashaqbeh, H. Investigation of carrageenan aerogel microparticles as a potential drug carrier. AAPS PharmSciTech 2018;19(1):36–47.
Patil A, Bjide S, Bookwala M, Soneta B, Shankar V, Almotairy A, et al. Stability of organoleptic agents in pharmaceuticals and cosmetics. AAPS PharmSciTech. 2018;19(1):36–47.
Acknowledgments
This work was supported by the Sahmyook University Research Fund in 2016 (no. RI22016007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Kang, C., Lee, JH., Kim, DW. et al. Preparation of Sustained Release Tablet with Minimized Usage of Glyceryl Behenate Using Post-Heating Method. AAPS PharmSciTech 19, 3067–3075 (2018). https://doi.org/10.1208/s12249-018-1128-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1128-7