Abstract
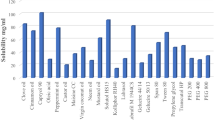
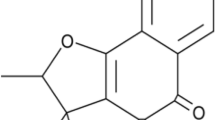
In this paper, a novel self-nanoemulsifying drug delivery system (SNEDDS) was used to improve the oral bioavailability in fasted state and diminish the food effect for rivaroxaban. Oil, surfactant, and co-surfactant were selected by saturated solubility study. IPM, Tween80, and 1,2-propanediol were finally selected as oil, surfactant, and co-surfactant, respectively. The pseudo-ternary-phase diagram was utilized to optimize the preliminary composition of SNEDDS formulation. The optimized rivaroxaban-SNEDDS formulation was selected by central composite design (CCD) of response surface methodology. Optimized SNEDDS formulation was evaluated for drug content, self-emulsifying time, droplet size, zeta potential, polydispersity index, Fourier transform-infrared (FTIR) spectroscopy, and transmission electron microscope (TEM). The drug dissolution profile compared to the commercial formulation Xarelto® (20 mg rivaroxaban) was determined in four different media (pH 1.2HCl, pH 4.5NaAc-HAc, pH 6.8PBS, and water). The result indicated that the SNEDDS formulation had successfully increased the drug solubility in four different media. A HPLC-MS method that indicated a high sensitivity, strong attribute, and high accuracy characteristic was built to measure the drug concentration in plasma. The fast/fed in vivo pharmacokinetics studies of SNEDDS formulation and Xarelto® were carried out in adult beagle dog, rivaroxaban with no food effect was achieved in SNEDDS formulation compared with Xarelto® in fed state. The result suggested that SNEDDS formulation in this study is useful to increase the oral bioavailability and diminish the food effect in fasted state.











Similar content being viewed by others
References
Yu LX, Straughn AB, Faustino PJ, Yang Y, Parekh A, Ciavarella AB, et al. The effect of food on the relative bioavailability of rapidly dissolving immediate-release solid oral products containing highly soluble drugs. Mol Pharm. 2004;1(5):357–62.
Ribas A, Zhang W, Chang I, Shirai K, Ernstoff MS, Daud A, et al. The effects of a high-fat meal on single-dose vemurafenib pharmacokinetics. J Clin Pharmacol. 2014;54(4):368–74.
Musson DG, Kramer WG, Foehr ED, Bieberdorf FA, Hornfeldt CS, Kim SS, et al. Relative bioavailability of sapropterin from intact and dissolved sapropterin dihydrochloride tablets and the effects of food: a randomized, open-label, crossover study in healthy adults. Clin Ther. 2010;32(2):338–46.
Thilakarathna SH, Rupasinghe HPV. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5(9):3367–87.
Okawara M, Hashimoto F, Todo H, Sugibayashi K, Tokudome Y. Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. Int J Pharm. 2014;472(1–2):257–61.
Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61(12):873–80.
Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(7):549–61.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60.
Gao Y, Li ZG, Sun M, et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36(10):1225–34.
Wan S, Sun Y, Qi X, Tan F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 2012;13(1):159–66.
Wang D, Chen G, Ren L. Preparation and characterization of the sulfobutylether-β-cyclodextrin inclusion complex of amiodarone hydrochloride with enhanced oral bioavailability in fasted state. AAPS PharmSciTech. 2017;18(5):1526–35.
Singh B, Beg S, Khurana RK, Sandhu PS, Kaur R, Katare OP. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit Rev Ther Drug Carrier Syst. 2014;31(2):121–85.
Li F, Song S, Guo Y, et al. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug Lornoxicam. Drug Deliv. 2014;22(4):1–11.
Xi J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10(1):172–82.
Cho HJ, Lee DW, Marasini N, Poudel BK, Kim JH, Ramasamy T, et al. Optimization of self-microemulsifying drug delivery system for telmisartan using Box–Behnken design and desirability function. J Pharm Pharmacol. 2013;65(10):1440–50.
Miao Y, Sun J, Chen G, et al. Enhanced oral bioavailability of lurasidone by self-nanoemulsifying drug delivery system in fasted state. Drug Dev Ind Pharm. 2015;42(8):1.
Zhu JX, Tang D, Feng L, Zheng ZG, Wang RS, Wu AG, et al. Development of self-microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev Ind Pharm. 2013;39(3):499–506.
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179–88.
Pandav G, Ganesan V. Efficacy of different block copolymers in facilitating microemulsion phases in polymer blend systems. Macromolecules. 2013;46(20):8334–44.
Patel N.D, Patel K.V, PanChal L.A, et al. An emerging technique for poorly soluble drugs: self emulsifying drug delivery system. International Journal of Pharmaceutical & Biological Archives. 2011;2(2):621–29.
Kyatanwar AU, Jadhav KR, Kadam VJ. Self micro-emulsifying drug delivery system (SMEDDS): review. J Pharm Res. 2015;1:75–83.
Kassem AM, Ibrahim HM, Samy AM. Development and optimisation of atorvastatin calcium loaded self-nanoemulsifying drug delivery system (SNEDDS) for enhancing oral bioavailability: in vitro and in vivo evaluation. J Microencapsul. 2017;34(3):319–33.
Pan G, Jia X, Wei H, et al. Comparison among several preparation methods for pseudo-ternary phase diagrams of pharmaceutical microemulsions. China Pharmacy. 2006;17(1):21–23.
Basalious EB, Abdallah AM. Phospholipid based self-nanoemulsifying self-nanosuspension (p-SNESNS) as a dual solubilization approach for development of formulation with diminished food effect: fast/fed in vivo pharmacokinetics study in human. Eur J Pharm Sci. 2017;109:244–52.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–61.
Miao Y, Chen G, Ren L, et al. Controlled release of glaucocalyxin—a self-nanoemulsifying system from osmotic pump tablets with enhanced bioavailability. Pharm Dev Technol. 2015;22(2):148–55.
Xu F, Wang LL, Shi ZQ, Chen F, Sun DM. Formulation optimization of Zuojin floating-bioadhesive pellets by central composite design-response surface methodology. J Chin Med Mater. 2015;38(9):1969–73.
Michaelsen MH, Wasan KM, Sivak O, et al. The effect of digestion and drug load on halofantrine absorption from self-nanoemulsifying drug delivery system (SNEDDS). AAPS J. 2015;18(1):180–6.
Thomas N, Holm R, Garmer M, et al. Supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. AAPS J. 2013;15(1):219–27.
Kumar R, Kumar S, Sinha VR. Evaluation and optimization of water-in-oil microemulsion using ternary phase diagram and central composite design. J Dispers Sci Technol. 2016;37(2):166–72.
Kang JH, Oh DH, Oh YK, Yong CS, Choi HG. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). Eur J Pharm Biopharm. 2012;80(2):289–97.
Kassem AA, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: design, optimization, in vitro, and in vivo, evaluation. J Mol Liq. 2016;218:219–32.
Miao Y, Chen G, Ren L, Ouyang P. Preparation and evaluation of ziprasidone-phospholipid complex from sustained-release pellet formulation with enhanced bioavailability and no food effect. J Pharm Pharmacol. 2016;68(2):185–94.
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391(1–2):203–11.
Zhang J, Li J, Ju Y, et al. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol Pharm. 2015;12(2):504–13.
Yadav P, Yadav E, Verma A, Amin S. In vitro characterization and pharmacodynamic evaluation of furosemide loaded self nano emulsifying drug delivery systems (SNEDDS). J Pharm Investig. 2014;44(6):443–53.
Rv T, Meijer J, Takusagawa S, et al. Development and validation of LC-MS/MS methods for the determination of mirabegron and its metabolites in human plasma and their application to a clinical pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci. 2012;887-888(7):102–11.
Verma S, Singh SK. LC-ESI-MS/MS estimation of loratadine-loaded self-nanoemulsifying drug delivery systems in rat plasma: pharmacokinetic evaluation and computer simulations by GastroPlus™. J Pharm Biomed Anal. 2016;124:10.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xue, X., Cao, M., Ren, L. et al. Preparation and Optimization of Rivaroxaban by Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Enhanced Oral Bioavailability and No Food Effect. AAPS PharmSciTech 19, 1847–1859 (2018). https://doi.org/10.1208/s12249-018-0991-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-0991-6