Abstract
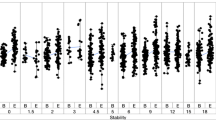
This article reports performance characteristics of the population bioequivalence (PBE) statistical test recommended by the US Food and Drug Administration (FDA) for orally inhaled products. A PBE Working Group of the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS) assembled and considered a database comprising delivered dose measurements from 856 individual batches across 20 metered dose inhaler products submitted by industry. A review of the industry dataset identified variability between batches and a systematic lifestage effect that was not included in the FDA-prescribed model for PBE. A simulation study was designed to understand PBE performance when factors identified in the industry database were present. Neglecting between-batch variability in the PBE model inflated errors in the equivalence conclusion: (i) The probability of incorrectly concluding equivalence (type I error) often exceeded 15% for non-zero between-batch variability, and (ii) the probability of incorrectly rejecting equivalence (type II error) for identical products approached 20% when product and between-batch variabilities were high. Neglecting a systematic through-life increase in the PBE model did not substantially impact PBE performance for the magnitude of lifestage effect considered. Extreme values were present in 80% of the industry products considered, with low-dose extremes having a larger impact on equivalence conclusions. The dataset did not support the need for log-transformation prior to analysis, as requested by FDA. Log-transformation resulted in equivalence conclusions that depended on the direction of product mean differences. These results highlight a need for further refinement of in vitro equivalence methodology.







Similar content being viewed by others
References
FDA. Guidance for industry. Statistical approaches to establishing bioequivalence. 2001. http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf Accessed 9 Jul 2017.
FDA. Draft guidance for industry. Bioavailability and bioequivalence studies for nasal aerosols and nasal sprays for local action. 2003. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070111.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on budesonide. 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM319977.pdf. Accessed 18 Sept 2017.
FDA. Draft guidance on azelastine hydrochloride and fluticasone propionate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM452689.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on aclidinium bromide. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM460918.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on albuterol sulfate. 2013. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM346985.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on beclomethasone dipropionate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM481768.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on budesonide; formoterol fumarate dihydrate. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM452690.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on ciclesonide (nasal). 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM319983.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on ciclesonide (inhalation). 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM481787.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on fluticasone furoate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495024.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on fluticasone furoate; vilanterol trifenatate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495023.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on fluticasone propionate. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM461051.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on fluticasone propionate; salmeterol xinafoate. 2013. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM367643.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on formoterol fumarate. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM461064.pdf Accessed 9 Jul 2017.
FDA. Draft guidance on formoterol fumarate; mometasone furoate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM481824.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on indacaterol maleate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495054.pdf Accessed 9 Jul 2017.
FDA. Draft guidance on ipratropium bromide. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM436831.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on levalbuterol tartrate. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM452780.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on mometasone furoate. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495387.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on mometasone furoate monohydrate. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM461141.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on umeclidinium bromide. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM520285.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on triamcinolone acetonide. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM520271.pdf. Accessed 9 Jul 2017.
FDA. Draft guidance on olopatadine hydrochloride. 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM520248.pdf. Accessed 9 Jul 2017.
Anderson S, Hauk W. Consideration of individual bioequivalence. Pharmacometrics. 1990;18:259–73.
Schall R, Luus HG. On population and individual bioequivalence. Stat Med. 1993;12:1109–24.
Morgan B, Strickland H. Performance properties of the population bioequivalence approach for in vitro delivered dose for orally inhaled respiratory products. AAPS J. 2013;16:89–100. https://doi.org/10.1208/s12248-013-9543-x.
Davit BM, Nwaka PE, Buehler GJ, Conner DP, Haidar SH, Patel DT, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–97. https://doi.org/10.1345/aph.1M141.
Tsong Y, Shen M. Parametric two-stage sequential quality assurance test of dose content uniformity. J Biopharm Stat. 2007;17:143–57. https://doi.org/10.1080/10543400601001527.
IPAC-RS. A parametric tolerance interval tests for improved control of delivered dose uniformity of orally inhaled and nasal drug products. https://ipacrs.org/assets/uploads/outputs/IPAC-RS_DDU_Proposal_15_Nov_2001_Final-pdf.pdf. Accessed 25 Oct 2017.
Thode HJ. Testing for normality. 1st ed. New York: Marcel Dekker; 2002.
Wasserstein RL, Lazar, NA. The ASA’s statement on p-values: context, process, and purpose. 2016;70:129–133.
IPAC-RS. Operating characteristic (OC) curves from simulation. Available at http://ipacrs.org/assets/uploads/outputs/Operating_Characteristic_(OC)_Curves_from_Simulation.pdf. Accessed 18 Sept 2017.
IPAC-RS. Summary data for operating characteristic curves from simulation. Available at http://ipacrs.org/assets/uploads/outputs/PBEsimresults_Sept2017.pdf. Accessed 18 Sept 2017.
FDA. Statistical information from the Jun 3 1999 draft guidance and statistical information for in vitro bioequivalence data posted on August 18, 1999. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070118.pdf. Accessed 11 Jul 2017.
Chiu ST, Tsai PY, Liu JP. Statistical evaluation of non-profile analyses for the in-vitro bioequivalence. J Chemom. 2010;24:617–25.
Welleck S. Testing statistical hypotheses of equivalence and noninferiority. 2nd ed. New York: CRC Press; 2010.
EMA. Reflection paper on statistical methodology for the comparative assessment of quality attributes in drug development. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/03/WC500224995.pdf. Accessed 7 Jul 2017.
Fuglsang A. Sample size estimation for the U.S. Food and Drug Administration’s in vitro population bioequivalence test of DPIs, MDIs, nasal sprays, and nebules. J Aerosol Med Pulm Drug Deliv. 2017;30:1–2. https://doi.org/10.1089/jamp.2017.1420.
Acknowledgements
The authors would like to acknowledge IPAC-RS member companies, board of directors, and all members of the PBE working group for supporting this work through discussions and by providing the data.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Equation 2 Full simulation model
Histograms of simulated data from Normal (left) and Log-normal (right) distributions with (a) 10% between-batch variance, and (b) 50% between-batch variance. Data were simulated from Eq. 2 in the Appendix, assuming mean (α) = 100 and σ2Total = 100, corresponding to relative standard deviation (RSD) = 10%, with three lifestages (n = 90) and no extreme values or lifestage effect
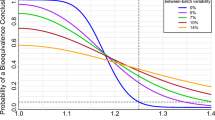
Operating characteristic (OC) curves for data simulated from a normal (black) or log-normal (red) distribution with 10% (solid) or 50% (dashed) between-batch variance, assuming three lifestages, no extreme values or lifestage effect, and equal RSD between test and reference products. The long-dashed gray line shows the “ideal” OC curve that marks the PBE equivalence region for the given settings
Rights and permissions
About this article
Cite this article
Morgan, B., Chen, S., Christopher, D. et al. Performance of the Population Bioequivalence (PBE) Statistical Test Using an IPAC-RS Database of Delivered Dose from Metered Dose Inhalers. AAPS PharmSciTech 19, 1410–1425 (2018). https://doi.org/10.1208/s12249-017-0941-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0941-8