Abstract
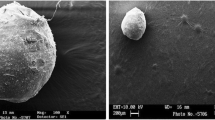
Lopinavir is a BCS Class IV drug exhibiting poor bioavailability due to P-gp efflux and limited permeation. The aim of this research was to formulate and characterize microspheres of lopinavir using thiolated xyloglucan (TH-MPs) as carrier to improve its oral bioavailability without co-administration of ritonavir. Thiomeric microspheres were prepared by ionotropic gelation between alginic acid and calcium ions. Interaction studies were performed using Fourier transform infrared spectroscopy (FT-IR). The thiomeric microspheres were characterized for its entrapment efficiency, T80, surface morphology, and mucoadhesion employing in vitro wash off test. The microspheres were optimized by 32 factorial design. The optimized thiomeric microsphere formulation revealed 93.12% entrapment efficiency, time for 80% drug release (T80) of 358.1 min, and 88% mucoadhesion after 1 h. The permeation of lopinavir from microspheres was enhanced 3.15 times as determined by ex vivo study using everted chick intestine and increased relative bioavailability over 3.22-fold over combination of lopinavir and ritonavir as determined by in vivo study in rat model.











Similar content being viewed by others
References
Bajaj H, Bisht S, Yadav M, Singh V. Bioavailability enhancement: a review. Int J Pharm Biol Sci. 2011;2:202–11.
Renukuntlaa J, Vadlapudib AD, Patelb A, Bodduc SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447:75–93.
Sham HL, Kempf DJ, Molla A, et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1998;42(12):3218–24.
Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS. 2001;15(1):F1–9.
Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retrovir. 1992;8(2):153–64.
Henderson LE, Bowers MA, Sowder RC, et al. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processing and complete amino acid sequences. J Virol. 1992;66(4):1856–65a.
Chandwani A, Shuter J. Lopnavir/ritonavir in treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4(5):1023.1033.
Sharma P, Garg S. Pure drug and polymer based nanotechnologies for improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62(4–5):491–502.
Jain S, Sharma JM, Jain AK, Mahajan RP. Surface stabilized lopinavir nanoparticles to enhance oral bioavailability without coadministartion of ritonavir. Nanomedicine. 2013;8(10):1639–55.
Khadka P, Ro J, Kim H, Kim I, et al. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9(6):304–16.
Kumar R, Sinha VR. Thiomer: a potential carrier for therapeutic delivery. React Funct Polym. 2013;73:1156–66.
Mishra A, Malhotra AV, Mater J. Tamarind xyloglucan: a polysaccharide with versatile potential. Chem. 2009;19:8528–36.
Gidley MJ, Lillford PJ, Rowlands DW, Lang P, Dentini M, et al. Structure and solution properties of tamarind polysaccharide. Carbohydr Res. 1991;214:299–314.
Sumathi S, Ray AR. Release behavior of drugs from xyloglucan tablets. J Pharm Sci. 2002;5:12–8.
Mohan M, Sujitha H, Rao M, et al. A brief review on mucoadhesive microspheres. IJRRPAS. 2014;4(1):975–86.
Aji Alex MR, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011;42(1–2):11–8.
Patel GM, Shelat PK, Lalwani AN. QBD based development of proliposome of lopinavir for improved oral bioavailability. Eur J Pharm Sci. 2016; doi:10.1016/j.ejps.2016.08.057.
Ravi PR, Rahul V, Vikas D, Aditya NM. A hybrid design to optimize preparation of lopinavir loadedsolid lipid nanoparticles and comparative pharmacokinetic evaluation with marketed lopinavir/ritonavir coformulation. J Pharm Pharmacol. 2014;66(7):912–26.
Rahmat D, Sakloetsakun D, Shahnaz G, Perera G, et al. Design and synthesis of novel cationic thiolated polymer. Int J Pharm. 2011;411:10–7.
Rahmat D, Muller C, Bernkop-Schnurch A. Thiolated hydroxyethyl cellulose: design and in vitro evaluation of mucoadhesion and permeation enhancing nanoparticles. Eur J Pharm Biopharm. 2013;83:149–55.
Galgette UC, Chaudhari PD. Preparation and evaluation of bioadhesive microspheres prepared by ion gelation method and effect of variables on quality of microspheres. Am J Pharm Tech Res. 2013;3(4):535–46.
Das MK, Senapati PC. Evaluation of furosemide loaded alginate microspheres prepared by ionotropic external gelation technique. Acta Pol Pharm. 2007;64(3):253–62.
Sonawane S, Bhalekar M, Shimpi S. Preparation and evaluation of microspheres of xyloglucan and derivative. Int J Biol Macromolec. 2014;69:499–505.
Lehr CM, Bowstra JA, Tukker JJ, Junginger HE, et al. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat—a comparative study with copolymers and blends based on poly(acrylic acid). J Control Release. 1990;13:51–62.
Donato EM, Martins LA, Froehlich PE, Bergold AM. Development and validation of dissolution test for lopinavir, a poorly water soluble drug, in soft gel capsule. J Pharm Biomed Anal. 2008;47(3):547–52.
Dixit P, Jain DK, Dumbwani J. Standardization of an ex vivo method for determination of intestinal permeability of drugs using everted rat intestine apparatus. J Pharmacol Toxicol Methods. 2012;65:13–7.
Bhalekar MR, Upadhaya PG, Reddy S, Kshirsagar SJ, Madgulkar AR. Formulation and evaluation of acyclovir nanosuspension for enhancement of oral bioavailability. Asian J Pharm. 2014;8:110–8.
Kumar K, Sudhakar M, Reddy P, Malleshwari P, Hafeez SK. RP-HPLC method development and validation for simultaneous estimation of lopinavir and ritonavir in dosage form and in plasma. Int J Pharm Sci Rev Res. 2014;3(9):1–8.
Dhakar RC, Maurya SD, Saluja V. From formulation variables to drug entrapment efficiency microspheres: a technical review. J Drug Deliv Ther. 2012;2:128–33.
Shivanarayana P, Kishore VS, Kumar PJ. Effect of crosslinking agent and polymer on the characterization of diltiazem hydrochloride loaded mucoadhesive microspheres. AJPTR. 2012;2(1):399–409.
Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity and reversibility. Pharm Res. 1994;11(8):1132–7.
Saremi S, Dinarvand R, Kebriaeezadeh A, Ostad SN, et al. Enhanced oral delivery of docetaxel using thiolated chitosan nanoparticles: preparation, in vitro and in vivo studies. Bio Med Res Int. 2013;2013:1–7.
Madgulkar AR, Bhalekar MR, Asgaonkar KD, Dikpati AA. Synthesis and characterization of novel mucoadhesive derivative of xyloglucan. Carbohydr Polym. 2016;135:356–62.
Yotsuyanagi T, Ohkubo T, Ohhashi T, Ikeda K. Calcium-induced gelatin of alginic acid and pH-sensitive reswelling of dried gels. Chem Pharm Bull. 1987;35:1555–63.
Acknowledgments
The authors acknowledge the financial assistance provided by Savitribai Phule Pune University under Board of College and University Development (BCUD) to undertake this research work. They are thankful to the Principal, AISSMS College of Pharmacy for providing necessary facilities to carry out the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madgulkar, A.R., Bhalekar, M.R. & Kadam, A.A. Improvement of Oral Bioavailability of Lopinavir Without Co-administration of Ritonavir Using Microspheres of Thiolated Xyloglucan. AAPS PharmSciTech 19, 293–302 (2018). https://doi.org/10.1208/s12249-017-0834-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0834-x