Abstract
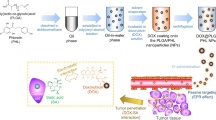
A novel multifunctional drug delivery system was fabricated by conjugating galactose-based polymer, methoxy-poly(ethylene glycol)-block-poly(6-O-methacryloyl-D-galactopyranose) (mPEG-b-PMAGP) with doxorubicin (DOX) via an acid-labile carbamate linkage. The mPEG-b-PMAGP-co-DOX nanoparticles were spherical in shape, and the diameter determined by dynamic light scattering (DLS) was 54.84 ± 0.58 nm, larger than that characterized by transmission electron microscopy (TEM). The in vitro drug release profiles were studied, and the release of DOX from the nanoparticles was pH-responsive. The cellular uptake behavior of free-DOX and mPEG-b-PMAGP-co-DOX nanoparticles by asialoglycoprotein (ASGP) receptor-positive cancer cell line (HepG2) and ASGP receptor-negative cancer cell lines (MCF-7 and A549 cells) was evaluated by confocal laser scanning microscopy (CLSM) and flow cytometry (FCM), respectively. The mPEG-b-PMAGP-co-DOX nanoparticles which contain galactose functional groups exhibited higher cellular uptake behavior via ASGP receptor-mediated endocytosis in HepG2 cells than in other two cancer cells. The in vitro cytotoxicity assay manifested that the mPEG-b-PMAGP-co-DOX nanoparticles exhibited higher anticancer efficacy against HepG2 cells than MCF-7 cells. These results indicated that the multifunctional mPEG-b-PMAGP-co-DOX nanoparticles possessing pH-responsible and hepatoma-targeting function have great potential to be used as a targeting drug delivery system for hepatoma therapy.









Similar content being viewed by others
References
Yang X, Grailer JJ, Rowland LJ, Javadi A, Hurley SA, Matson VZ, et al. Multifunctional stable and pH responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano. 2010;4:6805–17.
Lee YH, Park SY, Mok H, Park TG. Synthesis, characterization, antitumor activity of pluronic mimicking copolymer micelles conjugated with doxorubicin via acid-cleavable linkage. Bioconjugate Chem. 2008;19:525–31.
Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. PNAS. 2005;102:12962–7.
Fu F, Wu Y, Zhu J, Wen S, Shen M, Shi X. Multifunctional lactobionic acid-modified dendrimers for targeted drug delivery to liver cancer cells: investigating the role played by PEG spacer. ACS Appl Mater Interfaces. 2014;6:16416–25.
Ma S, Zhou J, Wali AR, He Y, Xu X, Tang JZ, et al. Self-assembly of pH-sensitive fluorinated peptide dendron functionalized dextran nanoparticles for on-demand intracellular drug delivery. J Mater Sci Mater Med. 2015;26:5550.
Chen KJ, Chaung EY, Wey SP, He Y, Xu X, Tang JZ, et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8:5105–15.
Luo Z, Ding X, Hu Y, Wu S, Xiang Y, Zeng Y, et al. Engineering hollow nanocontainer platform with multifunctional molecular machines for tumor-targeted therapy in vitro and in vivo. ACS Nano. 2013;7:10271–84.
Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7:5858–69.
Liu M, Huang G, Cong Y, Tong G, Lin Z, Yin Y, et al. The preparation and characterization of micelles from poly(γ-glutamic acid)-graft-poly(L-lactide) and the cellular uptake thereof. J Mater Sci Mater Med. 2015;26:187.
Wei M, Guo X, Tu L, Zou Q, Li Q, Tang C, et al. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int J Nanomedicine. 2015;10:5123–37.
Feng W, Nie W, He C, Zhou X, Chen L, Qiu K, et al. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake andbiodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl Mater Interfaces. 2014;6:8447–60.
Wang Z, Ma G, Zhang J, Lin W, Ji F, Bernards MT, et al. Development of zwitterionic polymer-based doxorubicin conjugates: tuning the surface charge to prolong the circulation and reduce toxicity. Langmuir. 2014;30:3764–74.
Hu X, Liu S, Huang Y, Chen X, Jing X. Biodegradable block copolymer-doxorubicin conjugates via different linkages: preparation, characterization, and in vitro evaluation. Biomacromolecules. 2010;11:2094–102.
Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–34.
Jung B, Jeong YC, Min JH, Kim JE, Song YJ, Park JK, et al. Tumor-binding prodrug micelles of polymer-drug conjugates for anticancer therapy in HeLa cells. J Mater Chem. 2012;22:9385–94.
Jin C, Qian N, Zhao W, Yang W, Bai L, Wu H, et al. Improved therapeutic effect of DOX-PLGA-PEG micelles decorated with bivalent fragment HAb18 F(ab′)2 for hepatocellular carcinoma. Biomacromolecules. 2010;11:2422–31.
Ulbrich K, Etrych T, Chytil P, Jelínková M, Ríhová B. Antibody-targeted polymer-doxorubicin conjugates with pH-controlled activation. J Drug Target. 2004;12:477–89.
Tu Y, Zhu L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J Control Release. 2015;212:94–102.
Shamay Y, Paulin D, Ashkenasy G, David A. E-selectin binding peptide-polymer-drug conjugates and their selective cytotoxicity against vascular endothelial cells. Biomaterials. 2009;30:6460–8.
Liu T, Li X, Qian Y, Hu X, Liu S. Multifunctional pH-disintegrable micellar nanoparticles of asymmetrically functionalized b-cyclodextrin-based star copolymer covalently conjugated with doxorubicin and DOTA-Gd moieties. Biomaterials. 2012;33:2521–31.
Fu C, Li H, Li N, Miao X, Xie M, Du W, et al. Conjugating an anticancer drug onto thiolated hyaluronic acid by acid lable hydrazone linkage for its gelation and dual stimuli-response release. Carbohydr Polym. 2015;128:163–70.
Luo Y, Bernshaw NJ, Lu ZR, Kopecek J, Prestwich GD. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm Res. 2002;19:396–402.
Pawar SK, Badhwar AJ, Kharas F, Khandare JJ, Vavia PR. Design, synthesis and evaluation of N-acetyl glucosamine (NAG)-PEG-doxorubicin targeted conjugates for anticancer delivery. Int J Pharm. 2012;436:183–93.
Tsai WB, Lai HY, Lee JL, Lo CW, Chen WS. Enhancement of the cytotoxicity and selectivity of doxorubicin to hepatoma cells by synergistic combination of galactose-decorated γ-poly(glutamic acid) nanoparticles and low-intensity ultrasound. Langmuir. 2014;30:5510–7.
Duncan R. Development of HPMA copolymer-anticancer conjugates: clinical experience and lessons learnt. Adv Drug Deliv Rev. 2009;61:1131–48.
Lv J, Sun H, Zou Y, Lo CW, Chen WS. Reductively degradable α-amino acid-based poly-(ester amide)-graft-galactose copolymers: facile synthesis, self-assembly, and hepatoma-targeting doxorubicin delivery. Biomater Sci. 2015;3:1134–46.
Ding YY, Han JT, Tian BC, Han J, Zhang J, Zheng H, et al. Hepatoma-targeting and pH-sensitive nanocarriers based on a novel D-galactopyranose copolymer for efficient drug delivery. Int J Pharm. 2014;477:187–96.
Wang Y, Hong CY, Pan CY. Galactose-based amphiphilic block copolymers: synthesis, micellization, and bioapplication. Biomacromolecules. 2013;14:1444–51.
Thapa B, Kumar P, Zeng H, Narain R. Asialoglycoprotein receptor-mediated gene delivery to hepatocytes using galactosylated polymers. Biomacromolecules. 2015;16:3008–20.
Sarika PR, James NR, Nishna N, Anil Kumar PR, Raj DK. Galactosylated pullulan-curcumin conjugate micelles for site specific anticancer activity to hepatocarcinoma cells. Colloids Surf B: Biointerfaces. 2015;133:347–55.
Hu FQ, Zhang YY, You J, Anil Kumar PR, Raj DK. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol Pharm. 2012;9:2469–78.
Duhem N, Danhier F, Pourcelle V, Schumers JM, Bertrand O, Leduff CS, et al. Self-assembling doxorubicin-tocopherol succinate prodrug as a new drug delivery system: synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjugate Chem. 2014;25:72–81.
Du JZ, Du XJ, Mao CQ, Wang J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc. 2011;133:17560–3.
Chen X, Parelkar SS, Henchey E, Schneider S, Emrick T. PolyMPC-doxorubicin prodrugs. Bioconjugate Chem. 2012;23:1753–63.
Acknowledgments
This work was financially supported by Shandong Provincial Natural Science Foundation, China (ZR2014HM096, ZR2013EMQ008, ZR2015HL123); Project of Medical and Health Science and Technology Development Program in Shandong Province (2014WS0484); and Science and Technology Plan Project of colleges and universities in Shandong Province (J14LM51).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yujie Sun and Jing Zhang are co-first authors.
Yujie Sun and Jing Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, Y., Zhang, J., Han, J. et al. Galactose-Containing Polymer-DOX Conjugates for Targeting Drug Delivery. AAPS PharmSciTech 18, 749–758 (2017). https://doi.org/10.1208/s12249-016-0557-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0557-4