Abstract
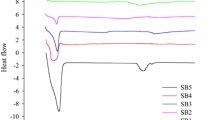
The stability of hydroxypropyl methylcellulose acetate succinate (HPMC-AS) and its potential incompatibility with active pharmaceutical ingredients (API) carrying hydroxyl group(s) were investigated in this research. HPMC-AS may undergo hydrolysis under harsh processing conditions with the generation of succinic acid and acetic acid, which can form ester bond(s) with the hydroxyl group(s) in API. In this case, the hot-melt extrusion (HME) product prepared from HPMC-AS and our model compound (compound A) was tested after heating at 140°C up to 5 h. The succinate esters of compound A and its epimer were found in the product, suggesting potential drug–excipient incompatibility during formulation development. In addition, dyphylline was also tested with HPMC-AS and the potential incompatibility was further confirmed.







Similar content being viewed by others
References
F. Tanno, Y. Nishiyama, H. Kokubo, and S. Obara. Evaluation of hydromellose acetate succinate (HPMCAS) as a carrier in solid dispersions. Drug Dev. Ind. Pharm. 30:9–17 (1999).
Shin-Etsu Chemical Co. Ltd., 1992. AQOAT product information brochure.
A. Serajuddin. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 88:1058–1066 (1999).
G. Amidon, H. Lennernäs, V. Shah, and J. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
T. Matsumoto, and G. Zografi. Physical properties of solid molecular dispersions of indomethacin with poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-vinyl-acetate) in relation to indomethacin crystallization. Pharm. Res. 16:1722–1728 (1999).
Z. Dong, A. Chatterji, H. Sandhu, D. Choi, H. Chokshi, and N. Shah. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int. J. Pharm. 355:141–149 (2008).
M. Fathy. Physicochemical characterization of ibuprofen/hypromellose acetate succinate solid dispersions. Mansoura J. Pharm. Sci. 22:224–237 (2006).
N. Wyttenbach, C. Birringer, J. Alsenz, and M. Kuentz. Drug–excipient compatibility testing using a high-throughput approach and statistical design. Pharmaceut. Dev. Tech. 10:499–505 (2005).
Q. Verloop, A. F. Marais, M. M. de Villier, and W. Liebenberg. Compatibility of sennoside A and B with pharmaceutical excipients. Pharmazie. 59:728–730 (2004).
G. Bruni, L. Amici, V. Berbenni, A. Marini, and A. Orlandi. Drug–excipient compatibilità studies. Search of interaction indicators. J. Therm. Anal. Calorim. 68:561–573 (2002).
J. L. Sims, J. A. Carreira, D. J. Carrier, S. R. Crabtree, L. Easton, S. A. Hancock, and C. E. Simcox. A new approach to accelerated drug–excipient compatibility testing. Pharmaceut. Dev. Tech. 8:119–126 (2003).
A. T. M. Serajuddin, A. B. Thakur, R. N. Ghoshal, M. G. Fakes, S. A. Ranadive, K. R. Morris, and S. A. Varia. J. Pharm. Sci. 88:696–704 (1999).
D. P. Elder. Organic impurities in drug products: origin, control and measurement. In R. J. Smith, and M. L. Webb (eds.), Analysis of Drug Impurities, Blackwell Publishing Ltd., Oxford, UK, 2007, pp. 21–46.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, Z., Choi, D.S. Hydroxypropyl Methylcellulose Acetate Succinate: Potential Drug–Excipient Incompatibility. AAPS PharmSciTech 9, 991–997 (2008). https://doi.org/10.1208/s12249-008-9138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-008-9138-5