Abstract
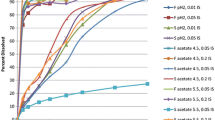
This study explored the in vivo performance of three oral ciprofloxacin formulations (oral solution, fast, or slow dissolving tablets) in beagle dogs. The in vivo absorption and dissolution behaviors, estimated with in silico mechanistic models, were compared to the results previously published in human volunteers. Six normal healthy male beagle dogs (five to completion) received three oral formulations and an intravenous infusion in a randomized crossover design. Plasma ciprofloxacin concentrations were estimated by tandem mass spectrometry detection. A mechanistic absorption model was used to predict the in vivo dissolution and absorption characteristics of the oral formulations. Canine ciprofloxacin absorption was constrained to the duodenum/jejunum. This absorption window was far narrower than that seen in humans. Furthermore, while substantial within-individual variability in drug absorption was seen in human subjects, a greater magnitude of variability was observed in dogs. For three sets of data, a lag time in gastric emptying was necessary to improve the accuracy of model-generated in vivo blood level profile predictions. In addition to species-associated dissimilarities in drug solubilization due to human versus canine differences in gastrointestinal fluid compositions, the far more rapid intestinal transit time and potential segmental differences in drug absorption needed to be considered during human-canine extrapolation of oral drug and drug product performance. Through the use of mechanistic models, the data generated in the human and canine studies contributed insights into some aspects of the interspecies differences to be considered when extrapolating oral bioavailability/formulation effect data between dogs and humans.










Similar content being viewed by others
Notes
Please note that in the write-up of study 1, we inadvertently labeled the base drug amount as 180.2 mg. The correct milligram free base amount of ciprofloxacin is 172 mg, as indicated in this write-up.
References
Akimoto M, Nagahata N, Furuya A, Fukushima K, Higuchi S, Suwa T. Gastric pH profiles of beagle dogs and their use as an alternative to human testing. Eur J Pharm Biopharm. 2000;49:99–102.
Lui CY, Amidon GL, Berardi RR, Fleisher D, Youngberg C, Dressman JB. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J Pharm Sci. 1986;75:271–4.
Martinez MN, Papich MG. Factors influencing the gastric residence of dosage forms in dogs. J Pharm Sci. 2009;98:844–60.
Papich MG, Martinez MN. Applying Biopharmaceutical Classification System (BCS) criteria to predict oral absorption of drugs in dogs: challenges and pitfalls. AAPS J. 2015;17:948–64.
Martinez M, Amidon G, Clarke L, Jones WW, Mitra A, Riviere J. Applying the biopharmaceutics classification system to veterinary pharmaceutical products. Part II. Physiological considerations. Adv Drug Deliv Rev. 2002;54:825–50.
Sutton SC. Companion animal physiology and dosage form performance. Adv Drug Deliv Rev. 2004;56:1383–98.
Arndt M, Chokshi H, Tang K, Parrott NJ, Reppas C, Dressman JB. Dissolution media simulating the proximal canine gastrointestinal tract in the fasted state. Eur J Pharm Biopharm. 2013;84:633–41.
Zhang H, Xia B, Sheng J, Heimbach T, Lin TH, He H, et al. Application of physiologically based absorption modeling to formulation development of a low solubility, low permeability weak base: mechanistic investigation of food effect. AAPS PharmSciTech. 2014;15:400–6.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11:225–37.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger MB, Brewster M, et al. In vivo methods for drug absorption—comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:99–151.
Martinez M, Mistry B, Lukacova V, Polli J, Hoag S, Dowling T, et al. Use of modeling and simulation tools for understanding the impact of formulation on the absorption of a low solubility compound: ciprofloxacin. AAPS J. 2016;18:886–97.
USP29 monographs, ciprofloxacin www.pharmacopeia.cn/v29240/usp29nf24s0_m17865.html.
Arakawa H, Shirasaka Y, Haga M, Nakanishi T, Tamai I. Active intestinal absorption of fluoroquinolone antibacterial agent ciprofloxacin by organic anion transporting polypeptide, Oatp1a5. Biopharm Drug Dispos. 2012;33:332–41.
Haslam IS, Wright JA, O’Reilly DA, Sherlock DJ, Coleman T, Simmons NL. Intestinal ciprofloxacin efflux: the role of breast cancer resistance protein (ABCG2). Drug Metab Dispos. 2011;39:2321–8.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50 Suppl 1:S41–67.
Hendriksen BA, Felix MV, Bolger MB. The composite solubility versus pH profile and its role in intestinal absorption prediction. AAPS PharmSci. 2003;5(1):E4.
Lu ATK, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharm Res. 1993;10:1308–14.
Mithani SD, Bakatselou V, TenHoor CN, Dressman JB. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm Res. 1996;13:163–7.
Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–80.
Mahar KM, Portelli S, Coatney R, Chen EP. Gastric pH and gastric residence time in fasted and fed conscious beagle dogs using the Bravo pH system. J Pharm Sci. 2012;101:2439–48.
Papich MG. Ciprofloxacin pharmacokinetics and oral absorption of generic ciprofloxacin tablets in dogs. Am J Vet Res. 2012;73:1085–91.
Abadía AR, Aramayona JJ, Muñoz MJ, Pla Delfina JM, Saez MP, Bregante MA. Disposition of ciprofloxacin following intravenous administration in dogs. J Vet Pharmacol Ther. 1994;17:384–8.
Abadia AR, Aramayona JJ, Muñoz MJ, Pla Delfina JM, Bregante MA. Ciprofloxacin pharmacokinetics in dogs following oral administration. Zentralbl Veterinarmed A. 1995;42:505–11.
Vance-Bryan K, Guay DR, Rotschafer JC. Clinical pharmacokinetics of ciprofloxacin. Clin Pharmacokinet. 1990;19:434–61.
Park MS, Okochi H, Benet LZ. Is ciprofloxacin a substrate of P-glycoprotein? Arch Drug Inf. 2011;4:1–9.
Drozdzik M, Gröer C, Penski J, Lapczuk J, Ostrowski M, Lai Y, et al. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol Pharm. 2014;11:3547–55.
Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol. 2005;70:695–9.
Haller S, Schuler F, Lazic SE, Bachir-Cherif D, Krämer SD, Parrott NJ, et al. Expression profiles of metabolic enzymes and drug transporters in the liver and along the intestine of beagle dogs. Drug Metab Dispos. 2012;40:1603–10.
Cho SM, Park SW, Kim NH, Park JA, Yi H, Cho HJ, et al. Expression of intestinal transporter genes in beagle dogs. Exp Ther Med. 2013;5:308–14.
Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003;43:211–27.
Kuentz M, Nick S, Parrott N, Röthlisberger D. A strategy for preclinical formulation development using GastroPlus as pharmacokinetic simulation tool and a statistical screening design applied to a dog study. Eur J Pharm Sci. 2006;27:91–9.
Kalantzi L, Persson E, Polentarutti B, Abrahamsson B, Goumas K, Dressman JB, et al. Canine intestinal contents vs. simulated media for the assessment of solubility of two weak bases in the human small intestinal contents. Pharm Res. 2006;23:1373–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Amin Rostami Hodjegan and Marilyn N. Martinez
Rights and permissions
About this article
Cite this article
Martinez, M.N., Mistry, B., Lukacova, V. et al. Exploring Canine-Human Differences in Product Performance. Part II: Use of Modeling and Simulation to Explore the Impact of Formulation on Ciprofloxacin In Vivo Absorption and Dissolution in Dogs. AAPS J 19, 712–726 (2017). https://doi.org/10.1208/s12248-017-0055-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0055-y