Abstract
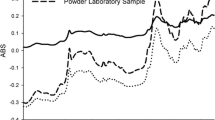
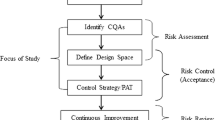
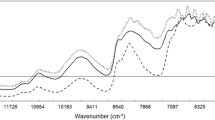
This article is the second of a series of articles detailing the development of near-infrared (NIR) methods for solid dosage-form analysis. Experiments were conducted at the Duquesne University Center for Pharmaceutical Technology to demonstrate a method for developing and validating NIR models for the analysis of active pharmaceutical ingredient (API) content and hardness of a solid dosage form. Robustness and cross-validation testing were used to optimize the API content and hardness models. For the API content calibration, the optimal model was determined as multiplicative scatter correction with Savitsky-Golay first-derivative preprocessing followed by partial least-squares (PLS) regression including 4 latent variables. API content calibration achieved root mean squared error (RMSE) and root mean square error of cross validation (RMSECV) of 1.48 and 1.80 mg, respectively. PLS regression and baseline-fit calibration models were compared for the prediction of tablet hardness. Based on robustness testing, PLS regression was selected for the final hardness model, with RMSE and RMSECV of 8.1 and 8.8 N, respectively. Validation testing indicated that API content and hardness of production-scale tablets is predicted with root mean square error of prediction of 1.04 mg and 8.5 N, respectively. Explicit robustness testing for high-flux noise and wavelength uncertainty demonstrated the robustness of the API concentration calibration model with respect to normal instrument operating conditions.
Similar content being viewed by others
References
US Food and Drug Administration. PAT: A Framework for Innovative Manufacturing and Quality Assurance, Guidance for Industry. US Food and Drug Administration, Washington, DC: 2003.
US Food and Drug Administration. Guidance Documents: Website of FDA Guidances. US Food and Drug Administration: 2004.
Box GEP, Jenkins GM, Reinsel GC.Time Series Analysis. Upper Saddle River, NJ: Prentice Hall; 1994.
Jackson JE, Mudholkar GS. Control procedures for residuals associated with principal components analysis.Technometrics. 1979;21:341–349.
Martens H, Naes T.Multivariate Calibration. New York, NY: John Wiley and Sons; 1989.
Mobley PR, Kowalski BR, Workman JJ Jr, Bro R. Review of chemometrics applied to spectroscopy: 1985–95, Part 2.Appl Spectrosc Rev. 1996;31:347–368.
Workman JJ Jr, Mobley PR, Kowalski BR, Bro R. Review of chemometrics applied to spectroscopy: 1985–95, Part I.Appl Spectrosc Rev. 1996;31:73–124.
Naes T, Martens H.Principal component regression in NIR analysis: viewpoints, background details, and selection of components.J Chemometric. 1988;2:155–167.
Wold S, Ruhe A, Wold H, Dunn WJ III. The collinearity problem in linear regression. the partial least squares approach to generalized inverses.SIAM J Sci Stat Comput. 1984;5:735–743.
Cogdill RP, Anderson CA, Delgado-Lopez M, et al. Process analytical technology case study: Part I. feasibility studies for quantitative NIR method development.AAPS Pharm Sci Tech. 2005;E263–273.
Ingle JD, Crouch SR.Spectrochemical Analysis. Englewood Cliffs, NJ: Prentice-Hall; 1988.
Williams P, Norris K, eds.Near-Infrared Technology in the Agricultural and Food Industries. 2nd ed. St Paul, MN: American Association of Cereal Chemists; 2001.
Drennen JK, Lodder RA. Nondestructive near-infrared analysis of intact tablets for determination of degradation products.J Pharm Sci. 1990;79:622–627.
Iyer M, Morris H, Drennen JK. Solid dosage form analysis by near infrared spectroscopy: Comparison of reflectance and transmittance measurements including the determination of effective sample mass.J Near Infrared Spectrosc. 2002;10:233–245.
Kirsch JD, Drennen JK. Determination of film-coated tablet parameters by near-infrared spectroscopy.J Pharm Biomed Anal. 1995;13:1273–1281.
Kirsch JD, Drennen JK. Near-infrared spectroscopy: applications in the analysis of tablets and solid pharmaceutical dosage forms.Appl Spectrosc Rev. 1995;30:139–174.
Kirsch JD, Drennen JK. Nondestructive tablet hardness testing by near-infrared spectroscopy: A new and robust spectral best-fit algorithm.J Pharm Biomed Anal. 1999;19:351–362.
Zeaiter M, Roger JM, Bellon-Maurel V, Rutledge DN. Rubustness of models developed by multivariate calibration. Part I: The assessment of robustness.Trends Anal Chem. 2004;23:157–170.
Bijlani VJ, Delgado-Lopez M, Adeyeye CM, Drennen JK. Near infrared spectroscopy for monitoring the hardness of roller compaction ribbons.NIR News. 2002;13:8–14.
Donoso M, Kildsig DO, Ghaly ES. Prediction of tablet hardness and porosity using near-infrared diffuse reflectance spectroscopy as a nondestructive method.Pharm Dev Technol. 2003;8:357–366.
Drennen JK, Voytilla RJ. Non-destructive tablet hardness testing: the effect of moisture on tablet hardness prediction.NIR News. 2002;13:12–13.
Bull CR. Compensation for particle size effects in near-infrared reflectance.Analyst. 1991;116:781–786.
Norris KH, Williams PC. Optimization of mathematical treatments of raw near-infrared signals in the measurement of protein in hard red spring wheat, I: Influence of Particle Size.Cereal Chem. 1984;61:158–165.
AACC. Near-Infrared Methods: Guidlines for Model Development and Maintenance. In:Approved Methods of the American Association of Cereal Chemists. Washington, DC: AACC Press; 1999; AACC Method 39.
Cogdill RP, Anderson CA, Drennen JK III. Integrated PAT Method Development. In:Process Analytical Technology, a supplement to Pharmaceutical Technology. Iselin, NJ: Advanstar Communications; 2004;29–34.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: October 6, 2005
The views presented in this article do not necessarily reflect those of the Food and Drug Administration.
Rights and permissions
About this article
Cite this article
Cogdill, R.P., Anderson, C.A., Delgado, M. et al. Process analytical technology case study: Part II. Development and validation of quantitative near-infrared calibrations in support of a process analytical technology application for real-time release. AAPS PharmSciTech 6, 38 (2005). https://doi.org/10.1208/pt060238
Received:
Accepted:
DOI: https://doi.org/10.1208/pt060238