Abstract
Background
Diabetic retinopathy (DR) is one of the leading causes of vision loss worldwide. For decades, 7-field 30-degree fundus imaging has been the gold standard for DR classification. The aim of this review article is to discuss how the advent of ultra-wide-field (UWF) fundus imaging has changed the management of proliferative diabetic retinopathy (PDR).
Main body
Current data suggests that UWF imaging, as compared to conventional Early Treatment Diabetic Retinopathy Study (ETDRS) fields, detects additional and more extensive PDR pathologies. DR lesions, captured by UWF imaging outside of ETDRS fields, likely carry prognostication value.
Conclusion
UWF imaging represents a major advancement in the detection and management of DR. It remains unclear whether, when and how patients, with PDR changes only peripheral to standard ETDRS fields, should be treated. A larger, prospective, randomized clinical trial is also needed to compare the efficacy of UWF image-guided targeted laser photocoagulation with that of conventional panretinal photocoagulation.
Similar content being viewed by others
Background
Diabetic retinopathy (DR) is one of the leading causes of vision loss in the world [1]. With the exponential increase in the incidence of diabetes mellitus, the prevalence and burden of DR is expected to increase dramatically world-wide in the coming decades [2]. Uncontrolled DR can eventually lead to severe vision loss and proliferative diabetic retinopathy (PDR), with features such as neovascularization of iris (NVI), neovascular glaucoma (NVG), neovascularization of disc (NVD), neovascularization elsewhere (NVE), vitreous hemorrhage (VH) and tractional retinal detachment (TRD). DR was first classified in 1968 by a group of experts in Airlie house, Virginia [3]. This classification was subsequently modified for use in the landmark trials of Diabetic Retinopathy Study (DRS) [4] and Early Treatment of Diabetic Retinopathy Study (ETDRS) [5] in the early 1980s and 1990s, respectively. This classification is based on findings within standardized, 7-field, 30-degree fundus photographs of the posterior pole, and has remained the gold standard for decades. In recent years, the advent of ultra-wide-field (UWF) imaging, defined by the Diabetic Retinopathy Clinical Research Network (DRCRnet) as a field-of-view of 100 degrees or more [6], has allowed for visualization of the far peripheral retina, areas that are beyond the field of view of traditional 7-field photographs (Fig. 1). Examples of machines capable of UWF imaging include Optos California® [Optos PLC, Dunfermline, United Kingdom] and Clarus® 500 [Carl Zeiss Meditec, Jena, Germany]. The aim of this review article is to discuss how these newer modalities of fundus imaging has and will change the management of PDR.
Sample ultra-wide-field imaging of a patient with proliferative diabetic retinopathy in the right (a) and left (b) eye. Early Treatment Diabetic Retinopathy Study (ETDRS) 7-field, 30-degree fundus images are superimposed in yellow circles. Corresponding fluorescein angiography of the right (c) and left (d) eye showed leakage from retinal neovascularization and non-perfusion
Detection, Prognostication and Treatment
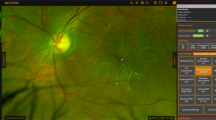
UWF fundus imaging allows for simultaneous documentation of a wide area of retina and has been shown to be more sensitive in detecting PDR, as compared to standard 7-field, 30-degree fundus imaging. Talks et al. [7]. showed that UWF photographs detected more NVE as compared to both standard DR screening protocol in England (two 45-degree image per eye) and 7-field 30-degree photographs. In 11.7% of the PDR eyes, retinal neovascularization was only detected outside the area covered on 7-field imaging. Wessel et al. [8]. evaluated patients with DR with UWF fluorescein angiography (FA) and superimposed standard 7-fields on these UWF FA images. As compared to standard 7-fields, UWF FA detected 3.9 times more non-perfusion and 1.9 times more NVE, both of which were statistically significant. Similar to findings by Talks et al., Wessel’s study showed that 16.7% of retinal neovascularization were only found outside the area covered on 7-field imaging. An example of PDR with retinal neovascularization only detected outside of ETDRS fields is shown in Fig. 2.
The ability of UWF imaging to capture additional DR features as compared to standard ETDRS fields also provides valuable prognostication information. Silva et al. [9]. showed that the presence of predominantly peripheral lesions (PPL), defined as any DR lesion located predominantly outside of standard ETDRS fields, increased the risk of DR progression and PDR development over 4 years by 3.2 and 4.7 times, respectively. These relationships remained statistically significant, even after adjusting for gender, diabetes type, diabetes duration, hemoglobin A1c levels, and baseline DR severity. In addition, a greater extent of PPLs at baseline also increased the risk of DR progression and PDR development in a statistically significant fashion. Oliver et al. [10]. showed that the presence of peripheral non-perfusion on UWF FA was associated with an increased risk of retinal neovascularization and macular ischemia, and these associations were statistically significantly. However, the caveat was that peripheral non-perfusion was definied as “capillary non-perfusion greater than 1 disc diameter in area outside the vascular arcades,” [10] meaning some of non-perfused areas were likely located within standard ETDRS fields. In addition, Oliver et al. described that the presence of late peripheral vessel leakage, defined as “late venous or arterial hyperfluorescence peripheral to the temporal vascular arcades,” [10] was associated with a statistically significant increase in the risk of retinal neovascularization.
The advent of UWF imaging also led to the practice of UWF-image-guided retinal laser photocoagulation, in which laser treatment was applied selectively to areas of non-perfusion as shown on UWF FA, in contrast to the conventional panretinal photocoagulation (PRP) technique that was first described in the DRS. Various authors [11, 12] have shown that UWF guided laser photocoagulation is effective in treating PDR and leading to regression of retinal neovascularization. Muqit et al. [11]. treated 28 treatment-naïve PDR eyes with targeted retinal photocoagulation. At 12 weeks, 76% of patients showed PDR regression, and 10 out of 28 eyes required repeat treatment. At 24 weeks, UWF FA showed complete disease regression and partial disease regression in 37% and 33% of eyes, respectively. As follow up, Muqit et al. [13]. carried out a pilot randomized trial, which compared targeted retinal photocoagulation with conventional PRP. At 12 weeks, between the UWF-guided targeted retinal photocoagulation group and the standard PRP group, there was no statistical difference in the change in visual acuity and the rate of PDR regression. Specifically, in the targeted retinal photocoagulation group, 60% and 10% of eyes showed partial and complete PDR regression, respectively. In the standard PRP group, 70% and 20% of eyes showed partial and complete PDR regression, respectively. However, the study only involved 30 eyes, and was likely under-powered to detect a difference between the two groups.
There are 2 major limitations to the use of UWF imaging. First, eyelid and eyelash artifacts (see upper left corner of Fig. 1a and lower left corner of Fig. 1b) are frequently encountered. Second, there is image magnification and distortion compared to the original scale, especially in the peripheral aspects of a UWF image where a lesion can be magnified in an exponential manner [14]. Therefore, any UWF imaging study that involves quantification of lesion size and longitudinal evaluation of lesion size changes must take this into account.
Conclusion and future directions
In summary, UWF imaging represents a major advancement in the detection and management of DR. Specifically, current data suggests that UWF imaging, as compared to conventional ETDRS fields, detects additional and more extensive PDR pathologies. DR lesions, captured by UWF imaging outside of ETDRS fields, likely carry prognostication value in terms of PDR development. These observations will be further evaluated by the DRCR.net Protocol AA, an on-going, prospective, observational, longitudinal study that aims to investigate whether UWF imaging, as compared to standard ETDRS fields, improves our ability to assess DR and predict DR progression over time. It will be interesting to see if the DRCRnet will recommend incorporating peripheral findings on UWF imaging into DR severity grading. In terms of treatment, the natural history of patients, with PDR changes only peripheral to standard ETDRS fields, will need to be elucidated, and it remains unclear whether, when and how these patients should be treated. In addition, a prospective, randomized clinical trial with a larger sample size will be needed to more definitively compare the efficacy of UWF image-guided targeted retinal photocoagulation versus that of conventional PRP.
Availability of data and materials
Not applicable.
Abbreviations
- DR:
-
diabetic retinopathy
- PDR:
-
proliferative diabetic retinopathy
- NVI:
-
neovascularization of iris
- NVG:
-
neovascular glaucoma
- NVD:
-
neovascularization of disc
- NVE:
-
neovascularization elsewhere
- VH:
-
vitreous hemorrhage
- TRD:
-
tractional retinal detachment
- DRS:
-
Diabetic Retinopathy Study
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- UWF:
-
ultra wide field
- FA:
-
fluorescein angiography
- PPL:
-
predominantly peripheral lesion
- PRP:
-
panretinal photocoagulation
References
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36.
Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–31.
Goldberg MF, Jampol LM. Knowledge of diabetic retinopathy before and 18 years after the Airlie House Symposium on Treatment of Diabetic Retinopathy. Ophthalmology. 1987;94(7):741–6.
Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):1–226.
Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification ETDRS report number 10. Ophthalmology. 1991;98(5 Suppl):786–806.
Ghasemi Falavarjani K, Tsui I, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy. Vis Res. 2017;139:187–90.
Talks SJ, Manjunath V, Steel DH, Peto T, Taylor R. New vessels detected on wide-field imaging compared to two-field and seven-field imaging: implications for diabetic retinopathy screening image analysis. Br J Ophthalmol. 2015;99(12):1606–9.
Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785–91.
Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949–56.
Oliver SC, Schwartz SD. Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol. 2010;25(1–2):27–33.
Muqit MM, Marcellino GR, Henson DB, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91(3):251–8.
Reddy S, Hu A, Schwartz SD. Ultra wide field fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9–14.
Muqit MM, Young LB, McKenzie R, et al. Pilot randomised clinical trial of Pascal TargETEd Retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. Br J Ophthalmol. 2013;97(2):220–7.
Oishi A, Hidaka J, Yoshimura N. Quantification of the image obtained with a wide-field scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 2014;55(4):2424–31.
Acknowledgements
None.
About this supplement
This article has been published as part of International Journal of Retina and Vitreous, Volume 5 Supplement 1, 2019: Wide-field imaging in retina and vitreous diseases. The full contents of the supplement are available at https://journalretinavitreous.biomedcentral.com/articles/supplements/volume-5-supplement-1.
Funding
No funding was received for this work. The publication costs for this paper in the supplement were made possible with unconditional financial support from Optos. The sponsor had no input into the content of articles, which were independently prepared by the authors and have undergone the journal’s standard peer-review process.
Author information
Authors and Affiliations
Contributions
T.Y.A.L. and J.F.A. are instrumental in the drafting and critical revision of this manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, T.Y.A., Arevalo, J.F. Wide-field imaging in proliferative diabetic retinopathy. Int J Retin Vitr 5 (Suppl 1), 20 (2019). https://doi.org/10.1186/s40942-019-0170-2
Published:
DOI: https://doi.org/10.1186/s40942-019-0170-2