Abstract
Background
Autism spectrum disorder (ASD) is a complex developmental disorder characterised by impaired social interaction and communication, and restrictive and repetitive behaviour. Previous systematic reviews have traditionally assessed the prevalence of ASD on global or regional context, with very few meta-analyses at the country level. The objective of this study will be to systematically evaluate published and unpublished observational studies that present prevalence and comorbidity of ASD among children, adolescent and adult population in Spain.
Methods/design
We designed and registered a study protocol for a systematic review and meta-analysis of descriptive epidemiology data. Observational studies (cohort, cross-sectional) reporting the prevalence of ASD and conducted in a wide range of people (e.g. general population, outpatient and/or school settings) will be included. The primary outcome will be the prevalence of ASD. Secondary outcomes will be the prevalence of any physical or mental comorbidity in association with ASD. No limitations will be imposed on publication status, study conduct period, and language of dissemination. Comprehensive literature searches will be conducted in seven electronic databases (from January 1980 onwards), including PubMed/MEDLINE, EMBASE, Scopus, Web of Science, PsycINFO, IME—Spanish Medical Index and IBECS—Spanish Bibliographic Index of Health Sciences. Grey literature will be identified through searching dissertation databases, Google Scholar and conference abstracts. Two team members will independently screen all citations, full-text articles, and abstract data. Potential conflicts will be resolved through discussion. The study methodological quality (or bias) will be appraised using an appropriate tool. If feasible, we will conduct random effects meta-analysis of observational data. Prevalence estimates will be stratified according to gender, age and geographical location. Additional analyses will be conducted to explore the potential sources of heterogeneity (e.g. methodological quality, sample size, diagnostic criteria).
Discussion
This systematic review and meta-analysis of observational data will identify, evaluate and integrate the epidemiological knowledge underlying the prevalence of ASD in Spain. The results of this study will be of interest to multiple audiences including patients, their families, caregivers, healthcare professional, scientists and policy makers. Results will be published in a peer-reviewed journal. Implications for future epidemiological research will be discussed.
Systematic review registration
PROSPERO CRD42018090372
Similar content being viewed by others
Background
Autism spectrum disorder (ASD) is a complex developmental disorder characterised by early-onset deficits in social communication and reciprocal interactions as well as the presence of restricted, stereotypical behaviour [1,2,3,4]. ASD presents a set of behavioural problems that may produce functional impairment in social, school, or work performance and in the everyday activities of patients and their families [1, 2, 5, 6]. Pathogenesis of the disorder is not completely understood, but there may be many different causes that make a child more likely to have an ASD, including genetic predisposition, environmental and psychosocial factors [1,2,3,4].
The prevalence of ASD is now considered to be around 1% in many countries and regions worldwide [7,8,9,10,11], although estimates of 2% have been suggested in some published reports [7, 12]. Similar prevalence estimates have been reported in adults [13, 14]. ASD seems to affect more male than female individuals [15], and comorbidity is common (e.g. more than 50% may present concurrent physical or mental conditions) [1, 16,17,18,19,20,21]. Most recent global burden of disease estimates revealed 62.2 million people with ASD around the world in 2016 [6].
Systematic reviews of epidemiological data (whether at a national, regional or global levels) are important in the description of the geographical distribution of health problems [22, 23]. Systematic reviews of epidemiological data can also identify gaps in knowledge and inform future health care planning and research agendas [24]. Specifically, they can be useful to get more precise estimates of disease frequency, monitor trends and changes in disease burden over time, and contribute to the design of further studies (e.g. etiological and interventional studies). Previous systematic reviews and meta-analyses have traditionally assessed the prevalence of ASD on global or regional contexts [7, 8, 25,26,27,28], with very few examples of descriptive epidemiology meta-analyses conducted at the country level (e.g. India, China, Hong Kong and Taiwan) [29,30,31].
In recent years, several observational studies have been conducted in different geographical locations and population groups [7]. According to recent epidemiological studies, in Spain, the prevalence of ASD would be 0.61% in children aged 18 months to 3 years in the Canary Islands [32], 0.85% in children aged 0–14 years in Galicia [33], 0.92% in children aged 18 months to 3 years in Castile-León [34], and 2% in samples of children aged 3–6 years in Catalonia [35]. To the best of our knowledge, no previous systematic reviews and meta-analyses have evaluated ASD prevalence data in Spain. Therefore, it would be relevant to identify, select, and critically appraise the relevant epidemiological literature on ASD and to collect and analyse data from studies through integrated and iterative approaches.
The objective of this study will be to systematically evaluate published and unpublished observational epidemiological studies that present prevalence and comorbidity of ASD among children, adolescent and adult population in a Southwestern European country: Spain.
Methods
Protocol
This study protocol is part of an ongoing evidence synthesis project on the descriptive epidemiology and surveillance of neurodevelopmental disorders [36]. The present protocol has been registered within the PROSPERO database (registration number CRD42018090372) and is being reported in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) statement [37, 38] (see checklist in Additional file 1).
Information source and literature search
The primary source of literature will be a structured search of seven major electronic databases (from January 1980 onwards—considering the Diagnostic and Statistical Manual of Mental Disorders, Third Edition [DSM-III] was published in 1980): PubMed/MEDLINE, EMBASE, Scopus, Web of Science, PsycINFO, and also national databases including IME—Índice Médico Español [Spanish Medical Index] and IBECS–Índice Bibliográfico Español en Ciencias de la Salud [Spanish Bibliographic Index of Health Sciences]). The secondary source of potentially relevant material will be a search of the grey or difficult to locate literature, including two dissertation databases (TESEO—Base de datos de Tesis Doctorales [Spanish Data Base of Doctoral Thesis Dissertations] and ProQuest Dissertations and Theses Database), Google Scholar and conference abstracts from selected national or local symposia on mental health, neurology and paediatrics. We will perform hand-searching of the reference lists of included studies, relevant reviews, national clinical practice guidelines or other relevant documents. Content experts and authors who are prolific in the field will be contacted. The literature searches will be designed and conducted by the review team which includes two experienced health information specialists. Our main literature search will be peer-reviewed by a senior health information specialist using the Peer Review of Electronic Search Strategies (PRESS) checklist [39]. The search will include a broad range of terms and keywords related to autism, epidemiological studies and the geographical area ‘Spain’. For the section of geographic area, the search will be based on a previously validated filter to minimise potential bias regarding the indexing of geographical items [40]. This filter is constructed around three complementary approaches: (1) the term ‘Spain’ and its variants in various languages, (2) terms related mainly to region and province place names, and (3) acronyms for regional health services. A draft search strategy for PubMed/MEDLINE is provided in Additional file 2.
Eligibility criteria
Studies will be selected according to the following criteria: participants, condition or outcome(s) of interest, study design and context.
-
Participants (population): We will include studies involving children, adolescents and adult population (regardless of age or sex).
-
Condition or outcome(s) of interest: The primary outcome will be the prevalence of ASD indicating the number of people that have the disorder divided by the population number at a given point in time. This is often presented as a (prevalence) proportion. In this study protocol, we use the term ‘autism spectrum disorder’ to refer to both autistic disorder (e.g. the ‘classic’ definition of autism) and ‘other autism spectrum disorder combined’ (e.g. Asperger’s disorder; pervasive developmental disorders not otherwise specified, including atypical autism; Rett’s disorder; and childhood disintegrative disorder) as recommended elsewhere [7]. We will use author-reported definitions (according to accepted diagnostic criteria, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD) criteria: ICD-9: 299.00, 299.10, 299.80; ICD-10: F84). Secondary outcomes will be the prevalence of any comorbidity, indicating the existence of any distinct additional (physical or mental) condition in association with ASD (e.g. according to main DSM-IV or ICD-10 categories of diagnoses).
-
Study design and context: Eligible studies will be observational studies (cohort, cross-sectional or health surveys) reporting prevalence data using validated or non-validated tools and conducted in a wide range of people in the Spanish general population, outpatient (including data from administrative databases and registries) and/or school settings. Cross-sectional studies will be the most appropriate study design to determine the prevalence of ASD. Cross-sectional health surveys are typically used to estimate the point prevalence of common conditions of long duration and are generally less frequently used for rare diseases such as ASD. For cohort studies, only the first phase (cross-sectional) data will be considered. We will exclude studies in hospital/inpatient clinical settings because they are likely to be highly selected resulting in inaccurate estimations of the ‘true prevalence’ of the disorder (selection bias).
No limitations will be imposed on publication status (unpublished studies will be eligible for inclusion), study conduct period, and language of publication.
Screening and selection procedure
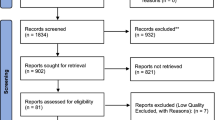
All articles identified from the literature search will be screened by two team members independently. First, titles and abstracts of articles returned from initial searches will be screened based on the eligibility criteria outlined above. Second, full texts will be examined in detail and screened for eligibility. Third, references of all considered articles will be hand-searched to identify any relevant report missed in the search strategy. Any disagreements will be resolved by discussion to meet a consensus, if necessary. A flow chart showing details of studies included and excluded at each stage of the study selection process will be provided [41].
Data collection
A data extraction form will be designed and used to extract equivalent information from each study report. Information of interest will include the following:
-
Study characteristics: study design, year of publication, journal, year (or period) of study conduct, sample size, setting (community, school or outpatient), geographical location of study conduct: North (Galicia, Asturias, Cantabria, Aragon, Basque Country, Navarre, La Rioja), Mediterranean (Balearics, Catalonia, Valencia), Centre (Castile-La Mancha, Castile-León, Madrid, Extremadura), and South-East (Andalusia, Murcia and Canary Islands); and other fields to capture data relevant to the assessment of study methodological quality (see risk of bias and methodological quality assessment subsection).
-
Participant characteristics: population sampled, age (e.g. mean with standard deviation, range) and gender (e.g. percentage of female participants).
-
Outcome results: definitions and assessment tools (e.g. DSM-III, DSM-IV, ICD-9, ICD-10, other), data collection method (e.g. self-complete, questionnaire, interview and examination, clinical records), prevalence estimates (e.g. number of subjects with the disorder, proportion and 95% confidence interval) and any prevalence estimates stratified by age, gender, severity or location. If outcome results (e.g. proportion and 95% confidence interval) are not directly provided and it is feasible, we will calculate them from the number of cases and sample size provided in each single study.
Data extraction forms will be piloted initially on a small number of included studies. Subsequently, each of the included studies will be abstracted by two team members, independently, and potential conflicts will be resolved through discussion. Authors of primary publications will be contacted for data clarifications or missing outcome data, as necessary.
Risk of bias and methodological quality assessment
The risk of bias of primary observational studies will be evaluated using a methodological quality critical appraisal checklist proposed in the Joanna Briggs Institute (JBI) systematic review methods manual [42, 43]. This methodological quality checklist for observational studies reporting prevalence data considers: sample representativeness, recruitment appropriateness, sample size, description of subjects and setting, coverage of data analysis, ascertainment and measurement of the condition, thoroughness of reporting statistical analysis, and identification and accountability of potential confounding factors/subgroups (see Additional file 3). We will provide a narrative summary of the risk of bias of the included studies, which will be supported by a table showing the results of the critical appraisal. Stars or points will be awarded for each quality item, and the highest quality studies will be awarded up to ten stars. Studies will be judged to be at low risk of bias (≥ 7 points), moderate risk of bias (4–6 points) or high risk of bias (< 4 points). The risk of bias for each observational study will be independently assessed by two reviewers. Discrepant scores will be resolved by discussion and consensus.
Methods for evidence synthesis
The data from each paper (e.g. study characteristics, context, participants, outcomes and findings) will be used to build evidence tables of an overall description of included studies. Crude prevalence estimates (number of cases/sample size) will be presented along with 95% confidence intervals.
If feasible and appropriate, prevalence data points from primary observational studies will be used to perform random effects meta-analyses. Since heterogeneity is expected a priori, we will estimate the pooled prevalence and its 95% confidence interval using the random effects model with logit transformation and back transformation as recommended elsewhere [8, 22]. The random effects model assumes the study prevalence estimates follow a normal distribution, considering both within-study and between-study variation. Forest plots will be used to visualise the extent of heterogeneity among studies. Prevalence estimates will be expressed as cases per 10,000 people [8, 31].
We will quantify statistical heterogeneity by estimating the variance between studies using I2 statistic [44]. The I2 is the proportion of variation in prevalence estimates that is due to genuine variation in prevalence rather than sampling (random) error. I2 ranges between 0% and 100% (with values of 0–25% and 75–100% taken to indicate low and considerable heterogeneity, respectively). We will also report Tau2 [45] and Cochran Q test [46] with a P value of < 0.05 considered statistically significant (heterogeneity).
Additional analyses
If sufficient studies are identified and data points are available, potential sources of heterogeneity will be investigated further by subgroup or meta-regression analyses according to baseline characteristics and methodological covariates. We plan to conduct analyses by gender (male vs female), age (e.g. children vs adolescent vs adult, mid-point of age range as continuous variable), severity (e.g. mild, moderate or severe), geographical location (e.g. North, Mediterranean, Centre and South-East), setting (e.g. community/school vs outpatient), sample size (e.g. < 1000, 1000–5000 or > 5000 participants), decade of publication (e.g. 1990, 2000 or 2010), study quality (e.g. low/moderate vs high-risk of bias), autism definition (e.g. autistic disorder vs other autism disorders combined), diagnostic system (e.g. DSM vs ICD criteria) and most recent diagnostic criteria (e.g. ‘DSM-IV or ICD-10’ vs ‘Not DSM-IV or ICD-10’). In addition, we will explore prevalence trends with gender variations (in terms of the female-to-male prevalence ratios) [15] and over time (with the year of publication as the explanatory variable) [1] using random effects meta-regression models [47]. Small study effects will be assessed by inspection of the funnel plots for asymmetry and with Egger’s test [48] and Begg’s test [49], with the results considered to indicate potential small study effects when P values < 0.10.
Software considerations
All analyses will be conducted in Stata version 15 (StataCorp LP, College Station, Texas, USA) [50, 51].
Ethics, dissemination and research integrity
No ethical approval is required for the performance of this study. The proposed systematic review and meta-analysis will be reported in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [41] and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) reporting guideline [52]. Any amendments made to this protocol when conducting the study will be outlined and reported in the final manuscript. Results will be disseminated through conference presentations and publication in a peer-reviewed journal. All data underlying the findings reported in the final manuscript will be deposited in a cross-disciplinary public repository—for example, the Open Science Framework (https://osf.io/) or Zenodo (https://zenodo.org/).
Patient and public involvement
One of the study protocol co-authors (AT) is the parent of a child with ASD and a member of a patient group (APRENEM Association for the Inclusion of People with ASD, Barcelona, Spain). We will evaluate whether the epidemiological studies included in the systematic review had any patient and/or public involvement [53, 54].
Discussion
The systematic review and meta-analysis of observational data presented in this protocol will identify, collect, evaluate and integrate the epidemiological knowledge underlying the prevalence of ASD in Spain. We are not aware of another systematic review and meta-analysis addressing this specific issue. In our opinion, this systematic review will help to establish the extent of the epidemiological evidence on ASD at a country level, in a reproducible and rigorous way.
The results of this study will be of interest to multiple audiences (including patients, their families, caregivers, healthcare professionals, researchers, scientists and decision makers). For example, the National Plan for Autism was issued in 2015 to develop specific measures to improve quality of life, promote awareness and identify and respond to the needs of those living with ASD [55]. The National Plan considered several strategic lines in terms of diagnosis, integrated care, health care, education, employment and research, among others. However, routine population estimates of ASD prevalence (based on the most updated systematic literature reviews) are not reported [55, 56]. Outputs of this analysis could be relevant to current policy questions.
There are several strengths and limitations of our planned systematic review methods. We will comprehensively evaluate epidemiological data (both published and unpublished) characterising the prevalence and comorbidity of ASD, exploring the extent of heterogeneity and potential biases in observational studies. We anticipate that we will identify knowledge gaps to be filled by new epidemiological research considering that the prevalence of neurodevelopmental disorders has been poorly covered in the literature [7, 57]. On this regard, implications for future epidemiological research will be discussed in the final manuscript. A key challenge is that based on knowledge from previous reviews on child mental health [7, 8, 25, 31, 57,58,59], we anticipate identifying studies using different study designs, populations, contexts and with a variable quality of reporting methods and results.
Finally, the availability of country-specific prevalence estimates of ASD over time will provide opportunities to undertake systematic assessments of the burden of disease and to promote evidence-based policy action [6, 60,61,62].
Abbreviations
- ASD:
-
Autism spectrum disorder
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- ICD:
-
International Classification of Diseases
- MOOSE:
-
Meta-analysis Of Observational Studies in Epidemiology
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRISMA-P:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Protocols
References
Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910. https://doi.org/10.1016/S0140-6736(13)61539-1.
Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4(4):339–46. https://doi.org/10.1016/S2215-0366(16)30376-5.
Blenner S, Reddy A, Augustyn M. Diagnosis and management of autism in childhood. BMJ. 2011;343:d6238. https://doi.org/10.1136/bmj.d6238.
Pilling S, Baron-Cohen S, Megnin-Viggars O, Lee R, Taylor C, Guideline Development Group. Recognition, referral, diagnosis, and management of adults with autism: summary of NICE guidance. BMJ. 2012;344:e4082. https://doi.org/10.1136/bmj.e4082.
Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721–8. https://doi.org/10.1001/jamapediatrics.2014.210.
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. https://doi.org/10.1016/S0140-6736(17)32154-2.
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–13. https://doi.org/10.1017/S003329171400172X.
Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child. 2006;91(1):8–15. https://doi.org/10.1136/adc.2004.062083.
Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289(1):49–55.
Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet. 2006;368(9531):210–5. https://doi.org/10.1016/S0140-6736(06)69041-7.
Idring S, Rai D, Dal H, Dalman C, Sturm H, Zander E, et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One. 2012;7(7):e41280. https://doi.org/10.1371/journal.pone.0041280.
Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168(9):904–12. https://doi.org/10.1176/appi.ajp.2011.10101532.
Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459–65. https://doi.org/10.1001/archgenpsychiatry.2011.38.
Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013–27. https://doi.org/10.1016/S2215-0366(15)00277-1.
Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466–74. https://doi.org/10.1016/j.jaac.2017.03.013.
Mazzone L, Ruta L, Reale L. Psychiatric comorbidities in asperger syndrome and high functioning autism: diagnostic challenges. Ann Gen Psychiatry. 2012;11(1):16. https://doi.org/10.1186/1744-859X-11-16.
Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–9. https://doi.org/10.1097/CHI.0b013e318179964f.
Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: an overview. Res Dev Disabil. 2007;28(4):341–52. https://doi.org/10.1016/j.ridd.2005.12.004.
Gillberg C, Billstedt E. Autism and Asperger syndrome: coexistence with other clinical disorders. Acta Psychiatr Scand. 2000;102(5):321–30. https://doi.org/10.1034/j.1600-0447.2000.102005321.x.
Schendel DE, Overgaard M, Christensen J, Hjort L, Jørgensen M, Vestergaard M, et al. Association of psychiatric and neurologic comorbidity with mortality among persons with autism spectrum disorder in a Danish population. JAMA Pediatr. 2016;170(3):243–50. https://doi.org/10.1001/jamapediatrics.2015.3935.
Lauritsen MB, Mors O, Mortensen PB, Ewald H. Medical disorders among inpatients with autism in Denmark according to ICD-8: a nationwide register-based study. J Autism Dev Disord. 2002;32(2):115–9.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–8. https://doi.org/10.1136/jech-2013-203104.
Flaxman AD, Vos T, CJL M, editors. An integrative metaregression framework for descriptive epidemiology. Seattle: University of Washington Press; 2015.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross-sectional study. PLoS Med. 2016;13(5):e1002028. https://doi.org/10.1371/journal.pmed.1002028.
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–79. https://doi.org/10.1002/aur.239.
Salhia HO, Al-Nasser LA, Taher LS, Al-Khathaami AM, El-Metwally AA. Systemic review of the epidemiology of autism in Arab Gulf countries. Neurosciences (Riyadh). 2014;19(4):291–6.
Hossain MD, Ahmed HU, Jalal Uddin MM, Chowdhury WA, Iqbal MS, Kabir RI, Chowdhury IA, Aftab A, Datta PG, Rabbani G, Hossain SW, Sarker M. Autism spectrum disorders (ASD) in South Asia: a systematic review. BMC Psychiatry. 2017;17(1):281. https://doi.org/10.1186/s12888-017-1440-x.
Kawa R, Saemundsen E, Lóa Jónsdóttir S, Hellendoorn A, Lemcke S, Canal-Bedia R, García-Primo P, Moilanen I. European studies on prevalence and risk of autism spectrum disorders according to immigrant status-a review. Eur J Public Health. 2017;27(1):101–10. https://doi.org/10.1093/eurpub/ckw206.
Chauhan A, Sahu JK, Jaiswal N, Kumar K, Agarwal A, Kaur J, Singh S, Singh M. Prevalence of autism spectrum disorder in Indian children: a systematic review and meta-analysis. Neurol India. 2019;67(1):100–4. https://doi.org/10.4103/0028-3886.253970 PubMed PMID: 30860104.
Wang F, Lu L, Wang SB, Zhang L, Ng CH, Ungvari GS, Cao XL, Lu JP, Hou CL, Jia FJ, Xiang YT. The prevalence of autism spectrum disorders in China: a comprehensive meta-analysis. Int J Biol Sci. 2018;14(7):717–25. https://doi.org/10.7150/ijbs.24063.
Sun X, Allison C, Matthews FE, Sharp SJ, Auyeung B, Baron-Cohen S, et al. Prevalence of autism in mainland China, Hong Kong and Taiwan: a systematic review and meta-analysis. Mol Autism. 2013;4(1):7. https://doi.org/10.1186/2040-2392-4-7.
Fortea Sevilla MS, Escandell Bermúdez MO, Castro Sánchez JJ. Estimated prevalence of autism spectrum disorders in the Canary Islands. An Pediatr (Barc). 2013;79(6):352–9. https://doi.org/10.1016/j.anpedi.2013.04.022.
Carballal Mariño M, Gago Ageitos A, Ares Alvarez J, Del Rio Garma M, García Cendón C, Goicoechea Castaño A, et al. Prevalence of neurodevelopmental, behavioural and learning disorders in Pediatric Primary Care. An Pediatr (Barc). 2017;(17):30417–4. https://doi.org/10.1016/j.anpedi.2017.10.007.
Canal-Bedia R, García-Primo P, Martín-Cilleros MV, Santos-Borbujo J, Guisuraga-Fernández Z, Herráez-García L, et al. Modified checklist for autism in toddlers: cross-cultural adaptation and validation in Spain. J Autism Dev Disord. 2011;41(10):1342–51. https://doi.org/10.1007/s10803-010-1163-z.
Morales P, Domènech-Llaberia E, Jané MC, Canals J. Mild autism spectrum disorders in preschool children: prevalence, co-occurrent symptoms and psychosocial development. Rev Psicopatol Psicol Clin. 2013;18(3):217–31.
Catalá-López F, Ridao M, Núñez-Beltrán A, Gènova-Maleras R, Alonso-Arroyo A, Aleixandre-Benavent R, Catalá MA, Tabarés-Seisdedos R. Prevalence and comorbidity of attention deficit hyperactivity disorder in Spain: study protocol for extending a systematic review with updated meta-analysis of observational studies. Syst Rev. 2019;8(1):49. https://doi.org/10.1186/s13643-019-0967-y.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1). https://doi.org/10.1186/2046-4053-4-1.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. https://doi.org/10.1136/bmj.g7647.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
Valderas JM, Mendivil J, Parada A, Losada-Yáñez M, Alonso J. Development of a geographic filter for PubMed to identify studies performed in Spain. Rev Esp Cardiol. 2006;59(12):1244–51.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute reviewer’s manual: The Joanna Briggs Institute; 2017. https://reviewersmanual.joannabriggs.org/ Accessed 22 Feb 2018.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53. https://doi.org/10.1097/XEB.0000000000000054.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions. Chichester: Wiley, 2008.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29.
Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J. 2008;8(4):493–519.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
StataCorp. Stata statistical software: release 15. College Station: StataCorp LP; 2017.
Palmer TM, Sterne JAC, Newton HJ, Cox NJ, editors. Meta-analysis in Stata: an updated collection from the Stata journal. 2nd ed. College Station: StataCorp LP; 2016.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. https://doi.org/10.1136/bmj.j3453.
Brett J, Staniszewska S, Simera I, Seers K, Mockford C, Goodlad S, et al. Reaching consensus on reporting patient and public involvement (PPI) in research: methods and lessons learned from the development of reporting guidelines. BMJ Open. 2017;7(10):e016948.
Estrategia Española en Trastornos del Espectro Autista. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2015. https://www.mscbs.gob.es/ssi/discapacidad/informacion/estrategiaEspanolaAutismo.htm Accessed 12 June 2019.
Estrategia Española en Trastornos del Espectro Autista. Madrid. Folleto resumen. 2016 http://www.autismo.org.es/sites/default/files/folleto_resumen_estrategia_espanola_autismo_version_impresion_personalizada_para_entidades.pdf Accessed 22 Feb 2018
Erskine HE, Baxter AJ, Patton G, Moffitt TE, Patel V, Whiteford HA, et al. The global coverage of prevalence data for mental disorders in children and adolescents. Epidemiol Psychiatr Sci. 2017;26(4):395–402. https://doi.org/10.1017/S2045796015001158.
Catalá-López F, Peiró S, Ridao M, Sanfélix-Gimeno G, Gènova-Maleras R, Catalá MA. Prevalence of attention deficit hyperactivity disorder among children and adolescents in Spain: a systematic review and meta-analysis of epidemiological studies. BMC Psychiatry. 2012;12:168. https://doi.org/10.1186/1471-244X-12-168.
Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Macías Saint-Gerons D, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS One. 2017;12(7):e0180355. https://doi.org/10.1371/journal.pone.0180355.
GBD 2010 Country Collaboration. GBD 2010 country results: a global public good. Lancet. 2013;381(9871):965–70. https://doi.org/10.1016/S0140-6736(13)60283-4.
GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–344. https://doi.org/10.1016/S0140-6736(17)32130-X.
Global Burden of Disease Child and Adolescent Health Collaboration, Kassebaum N, Kyu HH, Zoeckler L, Olsen HE, Thomas K, et al. Child and adolescent health from 1990 to 2015: findings from the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study. JAMA Pediatr 2017;171(6):573–592. doi: https://doi.org/10.1001/jamapediatrics.2017.0250.
Acknowledgements
Not applicable.
Ethical approval and consent to participate
Not applicable.
Availability data and materials
Not applicable.
Funding
FC-L and RT-S are funded by the CIBERSAM/Institute of Health Carlos III. MR and IH are partially funded by the Spanish Health Services Research on Chronic Patients Network (REDISSEC)/Institute of Health Carlos III. The funders were not involved in the design of the protocol or decision to submit the protocol for publication, nor will they be involved in any aspect of the conduct of the study.
Author information
Authors and Affiliations
Contributions
The study protocol was conceived by FC-L, with critical input from MR, IH, AN-B, RG-M, AA-A, AT, RA-B, MAC and RT-S. FC-L registered the protocol with the PROSPERO database and wrote the first draft of the protocol. MR, MAC and RT-S provided input into the design and edited the draft protocol. All authors commented on the paper for important intellectual content. FC-L accepts full responsibility for the finished paper and controlled the decision to publish. FC-L is the guarantor. All authors read and approved the final paper.
Authors’ information
FC-L is a PhD (Public Health) and MPH. MR is a PhD (Medicine) and MSc (Economics). IH is a PhD (Public Health) and BSc Hons (Psychology). AN-B is PsyD (Clinical Psychology) and MSc (Neurosciences). RG-M is a MSc (Demography). AA-A is a PhD (Information and Documentation) and MA. AT is a PhD (Public Health) and MSc (Statistics). RA-B is a MD and PhD (Medical Documentation). MAC is a MD (Child Psychiatry) and PhD (Medical Documentation). RT-S is a MD (Psychiatry) and PhD.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
PRISMA-P Checklist. (DOCX 32 kb)
Additional file 2:
Key terms for PubMed/MEDLINE search. (DOCX 28 kb)
Additional file 3:
Methodological Quality Checklist for Prevalence data. (DOCX 28 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Catalá-López, F., Ridao, M., Hurtado, I. et al. Prevalence and comorbidity of autism spectrum disorder in Spain: study protocol for a systematic review and meta-analysis of observational studies. Syst Rev 8, 141 (2019). https://doi.org/10.1186/s13643-019-1061-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-019-1061-1