Abstract
Background
Malaria control is highly dependent on the effectiveness of artemisinin-based combination therapy (ACT), the current frontline malaria curative treatment. Unfortunately, the emergence and spread of parasites resistant to artemisinin (ART) derivatives in Southeast Asia and South America, and more recently in Rwanda and Uganda (East Africa), compromise their long-term use in sub-Saharan Africa, where most malaria deaths occur.
Methods
Here, ex vivo susceptibility to dihydroartemisinin (DHA) was evaluated from 38 Plasmodium falciparum isolates collected in 2017 in Thiès (Senegal) expressed in the Ring-stage Survival Assay (RSA). Both major and minor variants were explored in the three conserved-encoding domains of the pfkelch13 gene, the main determinant of ART resistance using a targeted-amplicon deep sequencing (TADS) approach.
Results
All samples tested in the ex vivo RSA were found to be susceptible to DHA (parasite survival rate < 1%). The non-synonymous mutations K189T and K248R in pfkelch13 were observed each in one isolate, as major (99%) or minor (5%) variants, respectively.
Conclusion
The results suggest that ART is still fully effective in the Thiès region of Senegal in 2017. Investigations combining ex vivo RSA and TADS are a useful approach for monitoring ART resistance in Africa.
Similar content being viewed by others
Background
Prompt management of malaria cases remains a vital component of malaria control and elimination strategies [1]. Over the two last decades, artemisinin (ART)-based combination therapy (ACT) has contributed significantly to the reduction of malaria-related morbidity and mortality in sub-Saharan Africa [2]. Since 2006, the Senegalese National Malaria Control Programme (NMCP) has recommended artemether–lumefantrine (AL) as the first-line treatment for uncomplicated malaria [3]. However, recent reports of the emergence and expansion of partial resistance to ART (ART-R) in the Greater Mekong Subregion have endangered the long-term efficacy of ACT. Although ACT remains clinically and parasitologically effective in most African malaria endemic countries [4], the local emergences of ART-R in Rwanda and in Uganda, respectively reported in 2020 and 2021, are warning signals to the loss of ART efficiency [5,6,7,8]. As ART-R could independently emerge in western Africa or spread from eastern African ATR-R hotspots, the monitoring of Plasmodium falciparum parasites susceptibility to ART using the current clinical and biological tools is of utmost importance and must be implemented [4]. Clinically, ART-R is defined as delayed parasite clearance or Day-3 positive parasitaemia upon ART-based treatment [9]. Delayed clearance has been associated with decreased in vitro parasite susceptibility (survival rate ≥ 1%) expressed in the Ring-stage Survival Assay (RSA0–3 h) [10]. These clinical and biological phenotypes were later associated with non-synonymous mutations in the P. falciparum kelch13 gene (pfkelch13) [11]. Pfkelch13 encodes a 726-amino acid protein containing three structurally conserved domains: a coiled-coil-containing (CCC) domain, a Broad-Complex, Tramtrack and Bric a brac (BTB) domain, and a C-terminal kelch-repeat propeller domain where most of non-synonymous mutations associated with ART-R have been described [12]. Since then, a surveillance based on the detection of pfkelch13 single-nucleotide polymorphisms (SNPs) has been conducted in many malaria endemic settings. In the Greater Mekong Subregion, multiple ART-R pfkelch13 mutant parasites evolved concomitantly until a multidrug-resistant pfkelch13 C580Y lineage (named KEL1/PLA1) outcompeted the others and spread across Southeast Asia [13]. In Africa, this variant has been sporadically reported [14,15,16]. Rather, the pfkelch13 R561H mutant rapidly increased in frequency in Rwanda between 2014 and 2016 and 2018–2019 (from 8 to 22%, respectively) [17,18,19] and was associated with in vivo and in vitro ART-R [19]. Similarly, in Uganda, two pfkelch13 mutants (A675V and C469Y) were recently reported to be associated with in vivo and in vitro ART-R [6, 8, 20]. In Senegal, most investigations have looked for SNPs in the propeller-encoding domain of the pfkelch13 gene using conventional methods, such as PCR/Sanger sequencing [21,22,23,24,25,26], except for two studies that used pfkelch13 targeted-amplicon deep sequencing (TADS) [25, 26]. Although the authors did not detect validated or candidate ART-R pfkelch13 mutations [27], the TADS approach is particularly relevant to detect the presence of minor variants in P. falciparum isolates. Here, the pfkelch13 genotype was evaluated by TADS in 38 P. falciparum isolates collected in Thiès in 2017. The genetic variation was explored in the three conserved-encoding domains of pfkelch13 owing that one mutation in the BTB-encoding domain of pfkelch13 gene in a western African strain has been shown to confer ART-R [28].
Methods
Ethics
The National Ethics Committee for Health Research and the Ministry of Health of Senegal approved the protocol used for this study under number 00000169/MSAS/DPRS/CNERS (December 2, 2016). Written and informed consent was obtained from all participants, before participant recruitment and sample collection.
Study site
The study was conducted during the peak malaria season (September to December) in 2017 in Thiès, a city ~ 75 km southeast of Dakar (Senegal; 14° 47′ 26″ north, 16° 55′ 29″ west), an area belonging to the Sahelian facies defined by a short malaria seasonal transmission (< 4 months). In this region, the entomological inoculation rate (EIR) is low and varies from one year to another (0–20 infectious bites/person/year), estimated to be < 5 [29], and malaria is mainly transmitted by Anopheles arabiensis and Anopheles gambiae mosquito vectors. Malaria transmission is perennial and hypoendemic, and malaria incidence ranges between 5 and 15 per 1000 inhabitants [30].
Study design
Individuals who visited clinic of the Service de Lutte Antipaludique (SLAP) harbouring a P. falciparum infection only, confirmed by thick drop and thin smear; a parasite density between 1000 and 200,000 asexual forms/µL of blood; an absence of general danger signs or other signs of severe and complicated P. falciparum malaria as defined by the World Health Organization (WHO) [31]; a fever or history of fever within 48 h; absence of history of hypersensitivity reactions to the drugs assessed; living within 10 km of the health facility; and a provided informed consent (adult and/or equivalent minor), or legal guardian consent in the case of children, were enrolled. Patients with clinical malaria were treated with artemether–lumefantrine (AL, Coartem®), according to the treatment guideline of the Senegal National Malaria Control Programme (NMCP). For each patient, 5 mL vacutainer tubes of venous blood were collected for ex vivo RSA.
Parasite culture and ring-stage survival assay
Parasitaemia was estimated by microscopy examination on Giemsa-stained blood smears. Venous blood samples were then processed by eliminating plasma, leukocytes and anticoagulant from red blood cells (RBCs), washed twice in RPMI 1640 medium (Gibco, Life technologies). Parasitaemia were adjusted to 1% if greater by adding uninfected RBCs as previously described [10]. Then, 900 µL RBCs were loaded into wells, exposed to either 100 µL of 700 nM DHA (Sigma, reference D7439) or 0.1% of dimethyl sulfoxide (DMSO; Sigma, reference D8418) and cultivated at 37 °C in incubator for 6 h (conditions: 94% N2, 5% CO2, 1% O2). Finally, RBCs were washed and cultivated for 66 h. The proportions of viable parasites were estimated independently by two expert malaria microscopists on Giemsa-stained thin smears. The number of viable parasites that developed into ring/trophozoite stages were determined, pyknotic forms were excluded [32]. The average of the two counts was calculated. If any discrepancy was noted (either difference of parasite density of > 50%), slides were checked by a third independent reader, and parasite densities were calculated by averaging the two most close counts. Survival rates were calculated as the ratio of parasites in exposed and non-exposed cultures. Results were deemed as interpretable if the parasitaemia in the sample exposed to DMSO was higher than the initial parasitaemia at 0 h [33]. The P. falciparum ART-sensitive 3D7 strain was used as a negative control.
DNA extraction
DNA was extracted from the same whole blood sample used for the ex vivo RSA using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions.
Targeted-amplicon deep sequencing of pfkelch13
Extracted DNA samples were subjected to two separate overlapping PCRs covering the three conserved-encoding domains of the pfkelch13 gene (Jérôme Clain, personal communication). The first fragment covered both the CCC and BTB domains (5′-agatgcagcaaatctta-3′ and 5′-ttctacaccatcaaatcc-3) and measured 850 bp; and the second fragment covered from the end of the CCC domain to the end of the propeller domain (5′-aaaaagaaaaagaagaacataggaaa-3′ and 5′-tgtgcatgaaaataaatattaaagaag-3′) and measured 1,409 bp. Briefly, 1 µL of DNA was amplified with 0.25 µM of each primer, 0.2 mM of dNTP, 0.625 unit of GoTaq G2 Flexi DNA Polymerase (Promega, Madison, USA) and 2.5 mM and 3 mM of MgCl2 for the first and the second fragment, respectively. The cycling conditions were as follows: 3 min at 95 °C, then 40 cycles of 30 s at 95 °C, 30 s at 55 °C, 90 s at 68 °C and final extension 3 min at 68 °C. PCR products were detected using 1% agarose gel electrophoresis and SYBR Safe staining (Invitrogen, Waltham, USA). DNA from P. falciparum 3D7 strain and water were used as positive and negative controls, respectively.
Generated amplicons were then sequenced by next-generation sequencing. Briefly, PCR amplicons were fragmented with the Covaris S220 to about 150 bp, and libraries were constructed using the KAPA HyperPrep Library Preparation Kit (Kapa Biosystems, Woburn, MA) following the manufacturer’s protocol. Amplicons were purified with AMPure Agencourt XP beads (Beckman Coulter). Libraries quality and quantity control were assessed using Qubit® for concentration and Bio Analyser 2100 Agilent for fragment size. Libraries were pooled at approximately equal concentration and sequenced on an Illumina NextSeq 500 instrument (Illumina Inc, San Diego, CA, USA) to generate 150 bp paired-end reads at the GENOM’IC platform of Cochin Institute (Paris, France). Raw sequences were then demultiplexed and quality trimmed at a Phred score of 30. Primer sequences were trimmed from the 5′-end of the sequences to avoid primer bias in the sequenced fragments. Base calling was performed by comparing reads with a custom database consisting of the pfkelch13 sequence retrieved from the 3D7 reference genome. Bioinformatic analyses were performed using the CLC Genomics Workbench 22 software (Qiagen), using the following criteria: an allele was studied when its frequency was > 2%; the allele was considered as minor when the frequency was < 50%; otherwise, the allele was considered as major.
Results
Baseline characteristics
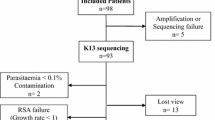
A total of 38 patients with uncomplicated P. falciparum malaria meeting the inclusion criteria were enrolled. The sex ratio (M/F) was largely dominated by males (36 males/2 females). The age of the participants ranged from 9 to 70 years (median of 21.5 years). Median weight was 59.5 kg and median body temperature was 38.5 °C. The median parasitaemia was 1.03%, ranging from 0.68 to 1.53% (Table 1).
Ex vivo RSA phenotype
Examination of blood smears and species-specific PCR revealed that all samples were P. falciparum monoinfections. Ex vivo RSA were successfully performed for the 38 tested samples. Survival rates showed the absence of surviving parasite to the 700 nM DHA pulse as for the 3D7 strain (0%) except for three isolates Th54, Th77 and Th95 (0.054%, 0.06% and 0.22% respectively) which were however less than the 1% threshold to be considered as associated with ART-R.
Pfkelch13 genotyping
Among the 38 samples successfully sequenced, two non-synonymous mutations (K189T and K248R) located outside of the propeller-encoding domain of pfkelch13 were detected (Table 2). Each mutation was observed in a single sample in major (99% for K189T) and minor (5% for K248R) proportions. The pfkelch13 K248R mutant was detected for the first time in Senegal (Table 3).
Discussion
ACT is the mainstay of treatment for uncomplicated malaria in malaria endemic regions [2]. Unfortunately, the emergence and the clonal expansion of pfkelch13 mutant parasites have been reported recently in Rwanda (R561H) and Uganda (C469Y and A675V) [5,6,7,8]. Therefore, the WHO recommends to closely monitor the susceptibility of P. falciparum to anti-malarial drugs and particularly to ART derivatives [4]. As in many sub-Saharan African countries, ACT has contributed significantly to the decline in malaria incidence and mortality over the past decade. In Senegal, considerable efforts have been done to reduce malaria morbidity and mortality mainly since 2006 when AL was introduced [3]. Consequently, the emergence of ART-resistant parasites is a major threat which can hinder malaria elimination.
Partial resistance to ART is associated with non-synonymous mutations in the pfkelch13 gene. However, the impact of most mutations on ART-R detected in field samples is largely unknown mainly due to the lack of association between the clinical phenotype (delayed parasite clearance) and the pfkelch13 genotype. The study presented here aims to fill this gap by combining ex vivo RSA with pfkelch13 genotyping. The ex vivo RSA estimates the susceptibility of P. falciparum to ART using parasite isolates freshly collected from patients with malaria. The ex vivo RSA has been previously used in studies conducted in Central [34] and East Africa [33, 35]. Four isolates from Uganda showed high (> 10%) survival rates, levels of which are reported to be closely associated with delayed parasite clearance following artesunate monotherapy [33]. The data presented here showed that none of the tested samples confer in vitro ART-R with a survival rate ≥ 1%, suggesting the absence of decrease susceptibility of Senegalese parasites to ART derivatives in 2017.
By using a TADS approach, two pfkelch13 non-synonymous mutations (K189T and K248R) were detected. The K248R mutation was observed in one sample in minor proportion (5%). The mutation is located in the CCC domain of pfkelch13, but the mutation is not likely related to ART-R since the survival rate was less than 1%. While K189T had already been reported in Africa [36, 37], the non-synonymous mutation K248R was detected for the first time in Senegal. The isolate carrying the K189T mutation had also a survival rate < 1%, confirming that this allele does not confer in vitro ART-R as previously observed in India [38]. Only three pfkelch13 wild-type isolates (Th54, Th77 and Th95) were found to have a survival rate above 0% (but still < 1%). To date, in vitro susceptibility to ART derivatives by RSA of P. falciparum isolates have been reported in Cameroon and Uganda. Two studies conducted in Cameroon [34, 35] showed the absence of pfkelch13 mutations associated with ART-R while one study in Uganda [33] reported high survival rates of isolates carrying the pfkelch13 A675V and C469Y mutations.
The study presented here has several limitations. First, this work included only 38 samples. Second, despite this low sample size, systematic ex vivo RSA is time consuming: parasites exposure to DHA is 6 h before washing the drug, several samples cannot be processed at the same time and the method requires skilled personnel, and all validation steps must be done by microscopy experts. Third, in vitro RSA (from culture-adapted parasites) was not performed to confirm the ex vivo survival rates. And fourth, the study was conducted only in one site in Senegal (Thiès) and no clinical data was collected.
Conclusion
This study shows that combining ex vivo RSA phenotype and pfkelch13 genotyping can be efficiently carried out to monitor ART-R, providing relevant data to malaria control programs on parasite susceptibility to ART. Particularly, TADS used here confirmed to be useful to detect the presence of minor alleles. This study showed that all P. falciparum isolates collected in Thiès (Senegal) in 2017 were susceptible to DHA. As recommended by the WHO [4], similar studies must be conducted in Senegal and other African countries to strengthen surveillance of anti-malarial drug efficacy and ART-R.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request. All relevant data are within the manuscript.
Abbreviations
- ACT:
-
Artemisinin-based combination therapy
- RSA:
-
Ring-stage Survival Assay
- TADS:
-
Targeted-amplicon deep sequencing
- PCR:
-
Polymerase chain reaction
- DNA:
-
Deoxyribonucleic acid
- DHA:
-
Dihydroartemisinin
- AL:
-
Artemether-lumefantrine
- ART:
-
Artemisinin
- ART-R:
-
Artemisinin resistance
- CCC:
-
Coiled-coil-containing
- BTB:
-
Broad-Complex, Tramtrack and Bric a brac
- SNP:
-
Single nucleotide polymorphism
- RBC:
-
Red blood cell
- NMCP:
-
National Malaria Control Programme
- WHO:
-
World Health Organization
References
WHO. Guidelines for malaria. WHO/UCN/GMP/2022.01 Rev. 2. Geneva. World Health Organization; 2022.
Nzoumbou-Boko R, Panté-Wockama CBG, Ngoagoni C, Petiot N, Legrand E, Vickos U, et al. Molecular assessment of kelch13 non-synonymous mutations in Plasmodium falciparum isolates from central African Republic (2017–2019). Malar J. 2020;19:191.
Programme National de Lutte contre le Paludisme (PNLP). Plan stratégique national de lutte contre le paludisme 2016–2020. Senegal: Dakar; 2015.
WHO. Tackling emerging antimalarial drug resistance in Africa. Geneva, World Health Organization, https://www.who.int/news/item/18-11-2022-tackling-emerging-antimalarial-drug-resistance-in-africa.
Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021;21:1120–8.
Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana SI, Yamauchi M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2021;385:1163–71.
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
Coppée R, Bailly J, Sarrasin V, Vianou B, Zinsou BE, Mazars E, et al. Circulation of an artemisinin-resistant malaria lineage in a traveler returning from East Africa to France. Clin Infect Dis. 2022;75:1242–4.
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67.
Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–9.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5.
Coppée R, Jeffares DC, Miteva MA, Sabbagh A, Clain J. Comparative structural and evolutionary analyses predict functional sites in the artemisinin resistance malaria protein K13. Sci Rep. 2019;9:10675.
Amato R, Pearson RD, Almagro-Garcia J, Amaratunga C, Lim P, Suon S, et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis. 2018;18:337–45.
Feng J, Li J, Yan H, Feng X, Xia Z. Evaluation of antimalarial resistance marker polymorphism in returned migrant workers in China. Antimicrob Agents Chemother. 2014;59:326–30.
Matrevi SA, Opoku-Agyeman P, Quashie NB, Bruku S, Abuaku B, Koram KA, et al. Plasmodium falciparum kelch propeller polymorphisms in clinical isolates from Ghana from 2007 to 2016. Antimicrob Agents Chemother. 2019;63:e00802–19.
Aninagyei E, Duedu KO, Rufai T, Tetteh CD, Chandi MG, Ampomah P, et al. Characterization of putative drug resistant biomarkers in Plasmodium falciparum isolated from ghanaian blood donors. BMC Infect Dis. 2020;20:533.
Uwimana A, Legrand E, Stokes BH, Ndikumana JLM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Bergmann C, van Loon W, Habarugira F, Tacoli C, Jäger JC, Savelsberg D, et al. Increase in Kelch 13 polymorphisms in Plasmodium falciparum, Southern Rwanda. Emerg Infect Dis. 2021;27:294–6.
Straimer J, Gandhi P, Renner KC, Schmitt EK. High prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis. 2022;225:1411–4.
Asua V, Conrad MD, Aydemir O, Duvalsaint M, Legac J, Duarte E, et al. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis. 2021;223:985–94.
Torrentino-Madamet M, Fall B, Benoit N, Camara C, Amalvict R, Fall M, et al. Limited polymorphisms in k13 gene in Plasmodium falciparum isolates from Dakar, Senegal in 2012–2013. Malar J. 2014;13:472.
Boussaroque A, Fall B, Madamet M, Camara C, Benoit N, Fall M, et al. Emergence of mutations in the K13 propeller gene of Plasmodium falciparum isolates from Dakar, Senegal, in 2013–2014. Antimicrob Agents Chemother. 2016;60:624–7.
Diallo MA, Yade MS, Ndiaye YD, Diallo I, Diongue K, Sy SA, et al. Efficacy and safety of artemisinin-based combination therapy and the implications of Pfkelch13 and pfcoronin molecular markers in treatment failure in Senegal. Sci Rep. 2020;10:8907.
Ahouidi A, Oliveira R, Lobo L, Diedhiou C, Mboup S, Nogueira F. Prevalence of pfk13 and pfmdr1 polymorphisms in Bounkiling, Southern Senegal. PLoS ONE. 2021;16:e0249357.
Talundzic E, Ndiaye YD, Deme AB, Olsen C, Patel DS, Biliya S, et al. Molecular epidemiology of Plasmodium falciparum kelch13 mutations in Senegal determined by using targeted amplicon deep sequencing. Antimicrob Agents Chemother. 2017;61:e02116–16.
Gaye A, Sy M, Ndiaye T, Siddle KJ, Park DJ, Deme AB, et al. Amplicon deep sequencing of kelch13 in Plasmodium falciparum isolates from Senegal. Malar J. 2020;19:134.
WHO. Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010–2019). https://www.who.int/publications-detail-redirect/9789240012813.
Paloque L, Coppée R, Stokes BH, Gnädig NF, Niaré K, Augereau JM, et al. Mutation in the Plasmodium falciparum BTB/POZ domain of K13 protein confers artemisinin resistance. Antimicrob Agents Chemother. 2022;66:e0132021.
Wong W, Griggs AD, Daniels RF, Schaffner SF, Ndiaye D, Bei AK, et al. Genetic relatedness analysis reveals the cotransmission of genetically related Plasmodium falciparum parasites in Thiès, Senegal. Genome Med. 2017;9:5.
Ndiaye D, Dieye B, Ndiaye YD, Van Tyne D, Daniels R, Bei AK, et al. Polymorphism in dhfr/dhps genes, parasite density and ex vivo response to pyrimethamine in Plasmodium falciparum malaria parasites in Thies, Senegal. Int J Parasitol Drugs Drug Resist. 2013;3:135–42.
WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:1–90.
Witkowski B, Menard D, Amaratunga C, Fairhurst R. Ring-stage survival assays (RSA) to evaluate the in-vitro and ex-vivo susceptibility of Plasmodium falciparum to artemisinins. Inst Pasteur Cambodge–National Inst Health Proced RSAv1; 2013.
Ikeda M, Kaneko M, Tachibana SI, Balikagala B, Sakurai-Yatsushiro M, Yatsushiro S, et al. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis. 2018;24:718–26.
Menard S, Tchoufack JN, Maffo CN, Nsango SE, Iriart X, Abate L, et al. Insight into k13-propeller gene polymorphism and ex vivo DHA-response profiles from Cameroonian isolates. Malar J. 2016;15:572.
Cooper RA, Conrad MD, Watson QD, Huezo SJ, Ninsiima H, Tumwebaze P, et al. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular assays. Antimicrob Agents Chemother. 2015;59:5061–4.
Kayiba NK, Yobi DM, Tshibangu-Kabamba E, Tuan VP, Yamaoka Y, Devleesschauwer B, et al. Spatial and molecular mapping of Pfkelch13 gene polymorphism in Africa in the era of emerging Plasmodium falciparum resistance to artemisinin: a systematic review. Lancet Infect Dis. 2021;21:e82–92.
Riloha Rivas M, Warsame M, Mbá Andeme R, Nsue Esidang S, Ncogo PR, Phiri WP, et al. Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine and polymorphism in Plasmodium falciparum kelch13-propeller gene in Equatorial Guinea. Malar J. 2021;20:275.
Das S, Saha B, Hati AK, Roy S. Evidence of artemisinin-resistant Plasmodium falciparum malaria in eastern India. N Engl J Med. 2018;379:1962–4.
Acknowledgements
We would like to acknowledge the African Center of Excellence for Genomics of Infectious Disease (ACEGID), the International Centre for Excellence in Malaria Research (ICEMR) project and the Parasitology and Mycology Laboratory Le Dantec Hospital. We thank Frédéric Ariey, Lucie Adoux, Adja Bousso Gueye and Daba Zoumarou for their contribution to this study and Jérôme Clain for sharing unpublished pfkelch13 PCR protocol. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding
The work was supported by the International Centers of Excellence for Malaria Research (ICEMR), West Africa (U19AI089696) and the Université Cheikh Anta Diop de Dakar. The study was also supported by the Institut Pasteur, Paris, the French Government (Agence Nationale de la Recherche), Laboratoire d’Excellence (LabEx) “French Parasitology Alliance for Health Care” (ANR-11-15 LABX-0024-PARAFRAP), the Cochin institute (ANR Chrono ANR-19-CE35-0009) and the University of Strasbourg through the Programme IdEX 2022 (DM).
Author information
Authors and Affiliations
Contributions
MSY, BD, DM and DN conceived and designed the study. IMN, YD and AMM collected the samples. AM, AF, AKB, MAD and KD performed ex vivo RSA and microscopy. FA, RC, JB, AM and CDL performed the sequencing. MSY, ABT, RC and FA performed data analysis and interpretation. MSY, RC, BD, TN, AG, AT, AM, ABT, MNG, MAD, KD and DN revised this manuscript. DM substantively revised this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Ethics Committee of the Ministry of Health in Dakar, Senegal 00000169/MSAS/DPRS/CNERS (December 2, 2016). Study participants, their parents, or guardians were informed about their rights, purpose, procedure, and benefit of the study and side effects of the procedure and written informed consent was obtained.
Consent for publication
The participants in this study consented for its publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yade, M.S., Dièye, B., Coppée, R. et al. Ex vivo RSA and pfkelch13 targeted-amplicon deep sequencing reveal parasites susceptibility to artemisinin in Senegal, 2017. Malar J 22, 167 (2023). https://doi.org/10.1186/s12936-023-04588-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04588-1