Abstract
Background
Malaria is a leading cause of mortality and morbidity in tropical countries, especially in sub-Saharan Africa. In Senegal, a control plan implemented in the beginning of the 2000s has enabled a substantial reduction of mortality and morbidity due to malaria. However, eradication of malaria requires a vaccine that protects against Plasmodium falciparum the deadliest species of the parasite that causes this disease. Plasmodium falciparum is characterized by an extensive genetic diversity that makes vaccine development challenging. In this study, the diversity of P. falciparum isolates was analysed from asymptomatic children residing in the district of Toubacouta, Senegal.
Methods
A nested PCR approach was used to perform genotyping of the msp-1 and msp-2 loci in samples from 87 asymptomatic children infected with P. falciparum, collected during a cross sectional survey in November and December 2010. Parasite densities in blood samples were determined by microscopic examination and statistical analyses were used to identify association of parasite genotype and parasitaemia.
Results
Genotyping was successful in 84/87 and 82/87 samples for msp-1 and msp-2, respectively. A strong genetic diversity was found with a total of 15 and 21 different alleles identified for msp-1 and msp-2, respectively. RO33 was the most frequent allelic family of msp-1 followed by MAD20, then by K1. Regarding msp-2 allelic families, 3D7 was more common than FC27. Multiple infections were predominant, since 69% and 89% of the samples genotyped for msp-1 and msp-2 showed more than one clone of P. falciparum with complexity of infection (COI) of 2.5 and 4.7, respectively. Expected heterozygosity (HE) was 0.57 and 0.55 for msp-1 and msp-2, respectively. Interestingly, polyclonal infections were significantly associated with higher parasitaemia.
Conclusions
The strong genetic diversity of P. falciparum clones and the association of polyclonal infection with high parasitaemia call for a multi-allelic approach in the design of vaccine candidates for efficient malaria eradication.
Similar content being viewed by others
Background
Malaria is a leading cause of mortality and morbidity in tropical countries. In 2016, more than 212 million of clinical cases and 429,000 deaths were reported worldwide. Sub-Saharan African countries support 92% of the global malaria burden, with children younger than 5 years, pregnant women, and non-immune visitors to endemic areas being people the most at risk of developing severe or fatal malaria [1]. In Senegal, 492,253 clinical cases and 526 deaths were reported in 2015. These figures represent a 12-times reduction compared to those from 2001, a substantial decrease that highlights the success of the malaria control programme that was implemented in Senegal in the beginning of the 2000s [2]. This malaria control programme was based on several approaches including widespread use of insecticide-treated mosquito nets (ITNs), rapid diagnostic testing (RDTs), treatment with artemisinin-based combination therapy (ACT), indoor residual spraying of insecticides (IRS), intermittent preventive treatment in pregnancy (IPTp) and seasonal malaria chemoprevention (SMC) in children under 10 years of age with sulfadoxine–pyrimethamine plus amodiaquine in areas of high seasonal malaria transmission [1]. However, despite this undisputable success, multiple factors can threaten malaria control efforts and compromise elimination including the emergence of drug-resistant parasite strains [3], insecticide-resistant mosquito vectors [4] as well as the weakness of the healthcare systems since malaria endemic countries are among the poorest in the world [5].
Achieving malaria elimination requires the development of an effective vaccine, especially against Plasmodium falciparum, which is the deadliest of the five human malaria parasites. A major obstacle toward this goal is the extensive genetic diversity of natural parasite populations that complicate the design of an effective vaccine against all P. falciparum strains. The difficulty related to P. falciparum genetic diversity has been clearly illustrated in clinical trials that tested different vaccine candidates. For example, the malaria vaccine combination B, designed as a three-component blood-stage product targeting the merozoite surface proteins MSP-1 (K1 parasite line) and MSP-2 (3D7), and the ring-infected erythrocyte surface antigen (RESA) was efficient against homologous parasites harboring the 3D7 allelic family of MSP-2, but failed to protect against those containing the FC27 allelic family, and was even associated with an increased rate of morbidity [6]. More recently, the malaria vaccine FMP2.1/AS02 (A), a recombinant protein based on the apical membrane antigen 1 (AMA1) from the 3D7 strain of P. falciparum did not provide significant protection against clinical malaria, but showed a strain-specific efficacy [7]. Moreover, a phase III clinical trial with RTS,S, the most advanced malaria vaccine candidate, showed a greater activity against parasites carrying the matching allele of the circumsporozoite protein than against other strains. In this last trial, less than 10% of the 5–17 months old children who were infected harbored parasites carrying the vaccine allele [8]. Collectively, these results point out the need to study P. falciparum polymorphism as an important step toward the identification of efficient malaria vaccine candidates.
The study presented here falls within the framework of preliminary investigations aimed at establishing a malaria clinical trial site in the district of Toubacouta (Fatick region, Senegal). It follows epidemiological [9], entomological [10] and immunological investigations undertaken in the same area. The objective is to characterize the genetic diversity of P. falciparum isolates from asymptomatic children in the selected study site by PCR-amplification of polymorphic regions of the two marker genes msp-1 and msp-2. The generated data are used to analyse the potential relationship between the levels of parasitaemia and specific parasite’s allelic types.
Methods
Study area
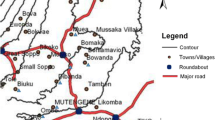
The study was conducted using samples from the rural community of Toubacouta situated in the region of Fatick (central-southern Senegal). The study area included eight villages: Haïdara, Daga Ndoup, Keur Ndianko, Nemanding, Passy Ndinderling, Keur Saloly Bouya, Keur Samba Gueye and Toubanding (Fig. 1). Malaria is mesoendemic in Toubacouta area with seasonal transmission occurring mainly during the rainy season between August and October, and with a peak in September [10]. The main vector belongs to the Anopheles gambiae complex. Malaria is mainly caused by P. falciparum with an entomological inoculation rate (EIR) of four infective bites per person from July to December 2011 in seven of the eight villages. Toubanding, the eighth village had an EIR of 30 infective bites per person owing to its proximity to the river Nema [10]. The population was estimated to 8000 inhabitants with 1500 children under 10 years (18.75%). Insecticide-treated nets were used by 52% of the population [10] and ACT was freely available to children.
Sample collection
A total of 1316 children under 10 years of age were enrolled in the study from November to December 2010. Children were examined by a medical doctor and clinical parameters were properly recorded. A venous blood sample was taken and used for blood typing, measurement of biological parameters, and for preparation of thick and thin blood smears for malaria diagnosis. A symptomatic case of malaria was defined as an axillary temperature ≥ 37.5 °C or any reported history of fever within the last 24 h confirmed by RDT [9]. These cases were excluded from our study. Plasma and blood pellets were separated and stored at − 20 °C for serological and molecular biology analyses, respectively.
Microscopic examination
Thick and thin smears were stained with 10% Giemsa for 25 min and microscopically examined for determination of parasite density and identification of species and developmental stages respectively. The number of parasites per 200 white blood cells (WBC) in thick-film was recorded and parasite density was estimated by counting the number of leucocytes by field examined and by arbitrarily considering that 8000 leucocytes were present in 1 µl of blood. At least 200 thick-film fields were examined before a slide was declared negative. Two experienced microscopists read the blood smears and in the case of a discrepancy, a third microscopist examined the slide. A slide was considered positive after two concordant readings by two different microscopists. Parasitaemias were classified into five levels including F1 (< 50 parasites/µl blood), F2 (50–499 parasites/µl), F3 (500–4999 parasites/µl), F4 (5000–49,999 parasites/µl) and F5 (≥ 50,000 parasites/µl) [11].
Extraction of parasite DNA
Genomic DNA (gDNA) was extracted from 100 μl packed red blood cells using QiaAmp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The DNA was recovered in 50 μl of elution buffer provided with the kit into a properly labelled Eppendorf tube. DNA concentration and purity were estimated using a NanoDrop Lite Spectrophotometer (Thermo Scientific). Extracted DNAs were stored at − 20 °C until used.
Genotyping of Plasmodium falciparum isolates
Genotyping of P. falciparum isolates was performed using a nested PCR approach targeting msp-1 and msp-2, two highly polymorphic region of the parasite’s genome as previously described [12]. All primers used were synthesized from Tib Molbiol (Germany). In the primary reaction, the primers used span the entire msp-1 block 2 and msp-2 block 3 respectively. The initial amplification was followed by individual nested PCR reactions using primers specific for K1, MAD20, and RO33 allelic types of msp-1, and FC27 and 3D7 allelic types of msp-2. In details, in the first PCR, 2.0 μl of DNA was amplified with 12.5 μl of 2× GMM (Go Taq Green Master Mix, Catalogue no M7113 Promega), 1 μl of forward primer (10 μM), 1 μl of reverse primer (10 μM) and sterile ultrapure water to a final volume of 25 μl. For the second round of PCR, 2.0 μl of first-round PCR products was amplified with 12.5 μl of 2× GMM (Go Taq Green Master Mix, M7113 Promega), 1.0 μl of forward primer (10 μM), 1.0 μl of reverse primer (10 μM) and sterile ultrapure water to a final volume of 25 μl. Primers and the reaction conditions are shown in Additional file 1: Table S1. Positive and negative controls were included in each set of reactions. Ten microliters of each PCR product were separated by electrophoresis on a 1.5% agarose gel that was stained with ethidium bromide. The size of the PCR products was estimated using a DNA ladder (1 kb Plus DNA ladder, Invitrogen). Amplified DNA was visualized by ultraviolet trans-illumination using an E-GEL IMAGER (Life Technologies). Gel photographs (Additional file 2: Fig. S1) were re-scored by visual comparison of DNA fragments and genotypes were identified according to band sizes for each individual sample. The size polymorphism for each allelic family was analysed assuming that one band represented one amplified PCR fragment derived from a single copy of P. falciparum msp-1 or msp-2 gene.
Statistical analysis
Asymptomatic malaria infection was defined as the presence of parasitaemia in the absence of symptoms or treatment. The complexity of infection (COI) also referred as multiplicity of infection (MOI) represented by the number of fragments per infected person, is the average number, for each gene, of distinct fragments per PCR positive sample. Expected heterozygosity (HE) was defined as the probability of being infected by two parasites with different alleles at a given locus. It is ranged between 0 and 1 was calculated by using the following formula: \({\text{H}}_{\text{E}} \, = \,\left[ {{\text{n}}/\left( {{\text{n}} - 1} \right)} \right] \, \left[ {\left( { 1- \sum {\text{pi2}}} \right)} \right]\), where n is the number of isolates sampled and pi is the allele frequency at a given locus [13]. Allelic frequency was estimated by calculating the percentage of fragments assigned to one family or family combination out of the total number of alleles detected for each gene [14]. Multiple infections (MI) or polyclonal infections corresponded to the proportion of isolates with more than one amplified PCR fragment. Comparison between mean parasite density of monoclonal and polyclonal infection was performed using the Student T test. Association between parasitaemia and allele frequencies was estimated using the Pearson correlation method. Differences were considered significant when a P value was < 0.05. All analyses were performed using R software (version 3.0.2).
Results
Demographic and parasitological data
Of the 1316 samples analysed by microscopic observation, 91 (6.91%) were positive for P. falciparum while no other Plasmodium species were detected. Four positive samples were excluded from the study due to the presence of fever at the time of blood collection. The mean age for the remaining 87 asymptomatic children was 6.5 years and the sex ratio (F/M) was 0.74 (Table 1). The mean parasite density was 8915.7 parasites/μl of blood, while the median was 960 parasites/μl with a range from 0 to 106,560 parasites/μl. These parasitaemias only referred to the amount of asexual blood stage parasites. Gametocytes presence was reported without quantification. Hence, a sample was considered positive with 0% asexual blood stage parasites if gametocyte presence was confirmed [15]. The genotyping success rate was 96.5% (84/87) and 94% (82/87) for msp-1 and msp-2, respectively.
Allelic diversity of Plasmodium falciparum msp-1 and msp-2 genes
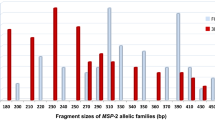
For msp-1 gene, 15 different alleles were identified including 6, 5 and 4 belonging to the K1, MAD20 and RO33 allelic families respectively. The total number of msp-1 alleles detected in all samples was 219. Allele frequencies were 40.2% (88/219), 31.1% (68/219), and 28.7% (63/219) for RO33, MAD20 and K1, respectively (Table 2). Expected heterozygosity (HE) was 0.67 for msp-1 loci. Of msp-1 positive samples, 69% (58/84) were polyclonal. The mean complexity of infection (COI) was 2.5. These polyclonal infections mostly involved trimorphic and dimorphic allelic family combinations: 27.4% (23/84) of K1/MAD20/RO33, 16.7% (14/84) of MAD20/RO33, 14.3% (12/84) of K1/RO33 and 5.9% (5/84) of K1/MAD20. The maximum number of genotypes belonging to the same allelic family detected in a single isolate was 3, 5, and 4, for K1, MAD20 and RO33, respectively.
For msp-2 gene, 21 different alleles were detected with 10 for 3D7 allelic family and 11 for FC27. Allelic family frequencies showed that the 3D7 allelic type was predominant (53.7%, 206/384) over FC27 (46.3%, 178/384) (Table 2). The prevalence of multiple infections (MI) was 89% (73/82). Expected heterozygosity (HE) was 0.50 for msp-2 loci. The prevalence of multiple infections (MI) was 89% (73/82). The mean complexity of infection was 4.7. The frequency of infections involving the two allelic families of msp-2 was 63% (52/82). The maximum number of genotypes to belong to the same allelic family detected in a single isolate was 5 and 7 for 3D7 and FC27, respectively.
Ranges of fragment sizes for msp-1 and msp-2 allelic families are shown in Additional file 3: Table S2.
Parasitaemia is higher in polyclonal than in monoclonal asymptomatic infection by Plasmodium falciparum
Parasitaemias were compared in samples with polyclonal versus monoclonal infections. The analyses were performed after removal of eight samples that were found to be outliers (extreme 5% values showed as dots of boxplot in Additional file 4: Fig S2). Mean parasite density was significantly higher (Student T test, P = 0.012) in polyclonal (mean density = 3347 parasites/μl) than in monoclonal infection (mean density of 1229 parasites/μl) for msp-1 positives isolates (Fig. 2). A similar result was observed with msp-2 (Fig. 2), with significantly higher parasitaemia (Student T test, P = 0.00008) in polyclonal (mean density of 3092 parasites/μl) than in monoclonal infections (561 parasites/μl).
msp-1 and msp-2 allelic families influence parasitaemia in asymptomatic Plasmodium falciparum infection
To further analyse the effect of polyclonal infection on parasitaemia, the influence of specific allele combinations on parasite density was assessed. For this purpose, the samples were classified according to their parasite density and five levels of parasitaemia were set. Analysis of the relationship between msp-1 allelic family combinations and parasitaemia (Fig. 3) showed a significant positive association between trimorphic infections K1/MAD20/RO33 and parasitaemia (r2 = 0.93; P = 0.007). In contrast, parasitaemia were significantly and negatively correlated with MAD20 monoallelic infections (r2 = 0.93; P = 0.008). Regarding msp-2, FC27 monoallelic infections were negatively associated with parasitaemia (r2 = 0.82; P = 0.034).
Discussion
Preliminary studies for a clinical trial site for malaria have been undertaken in the district of Toubacouta (Fatick region, Senegal). Eight villages were involved. The genetic diversity of P. falciparum, the only species diagnosed, was analysed in samples from asymptomatic children collected through a cross-sectional survey. An important allelic diversity was observed with 15 and 21 different alleles, and 69% and 89% of multiple infections, for msp-1 and msp-2 genes, respectively. The high genetic diversity in our study is in agreement with previous findings on asymptomatic children in the mesoendemic malaria site of Niakhar in Senegal. In Niakhar, genotyping with the msp-2 polymorphic marker revealed a range from 2 to 7 different fragments per carrier with 64% of multiclonal infections [16]. Similar results were observed 16 years ago in Ndiop in asymptomatic children [14]. Comparison of genetic diversity of P. falciparum isolates in Ndiop (1994) [14] and our study area (2010) in the nearby locality shows a stable genetic diversity over time. A high level of diversity persists despite a 12 times difference in transmission levels (63 infected bites per person per 4 months in 1994 compared to four infected bites per person per 3 months in 2010) between the two periods as a result of malaria control interventions [17]. This observation underlines that P. falciparum genetic diversity does not only rely on parasite transmission rate. Thus, it has been demonstrated that the genotypic profile changes within hours or days in asymptomatic infections [18].
Unlike genetic diversity, complexity of infection (COI) is more commonly associated with the level of malaria transmission. In line with this observation, Konate et al. [19] found that COI was twice higher in the holoendemic area of Dielmo than in the mesoendemic village of Ndiop. Low COI was also associated with low transmission rate in Malaysia [13]. Vafa et al. [16] highlighted COI variation within the same year according to transmission period. In this study, COI is consistent with results from mesoendemic regions for both msp-1 and msp-2 markers [14, 20, 21].
Association between the number of clones per sample and the level of parasitaemia has been investigated. The results show that polyclonality is likely to be associated with high parasitaemia. This is consistent with previous studies [16, 21,22,23]. On the one hand, parasite density is attributed a major role in malaria physiopathology and some authors suggest that it might be used as a marker for morbidity and mortality associated with this disease [24, 25]. On the other hand, it is considered as the centerpiece of malaria immunity. In this regard, it has been shown that asymptomatic polyclonal carriage may protect against clinical malaria [26, 27]. Although the mechanism of this partial protection remains unclear, it is probably by maintaining protective immune responses to asexual blood stage parasites [21, 28, 29].
Moreover, this study emphasizes the significant positive correlation between frequencies of trimorphic infections K1/MAD20/RO33 of msp-1 gene and parasitaemia (Fig. 3). Although the representative alleles of this combination have to be identified, these preliminary results point out the necessity of multivalent vaccine candidates to overcome antigenic diversity [30].
This study has some technical limitations. Firstly, PCR cannot discriminate between alleles of differing sequences with similar size and can thus underestimate the number of distinct alleles [30, 31]. Secondly, polymorphism of sub-microscopic infections is not taken into account in this study. Despite these limitations, the results provide an insight on the genetic diversity of P. falciparum clones circulating in Toubacouta, Senegal.
Conclusions
Despite the general decline in malaria prevalence observed following the implementation of various malaria control strategies, P. falciparum strains are substantially circulating in this study site and display a high genetic diversity. The level of this diversity is comparable to that found in malaria endemic and mesoendemic areas, therefore, making the site interesting for potential vaccine and therapeutic clinical trials. The positive correlation between msp-1 trimorphic infection and parasitaemia suggests the use of field information on genetic diversity as starting blocks for designing new malaria vaccines.
Abbreviations
- RDT:
-
rapid diagnostic test
- IRS:
-
indoor residual spraying
- LLIN:
-
long-lasting insecticide-treated nets
- ACT:
-
artemisinin-based combination therapy
- IPTp:
-
intermittent preventive treatment in pregnancy
- SMC:
-
seasonal malaria chemoprevention
- msp1 :
-
merozoite surface protein 1
- msp2 :
-
merozoite surface protein 2
- EIR:
-
entomological inoculation rate
- DNA:
-
deoxyribonucleic acid
- PCR:
-
polymerase chain reaction
- UV:
-
ultraviolet
- COI:
-
complexity of infection
- MOI:
-
multiplicity of infection
- MI:
-
multiple infections
- bp:
-
base pairs
- HE :
-
expected heterozygosity
References
WHO. World malaria report 2016. Geneva: World Health Organization; 2016.
PNLP. Bulletin Epidemiologique Annuel du Paludisme au Senegal. Dakar: Programme National de Lutte contre le Paludisme; 2015. p. 24.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23.
Kristan M, Lines J, Nuwa A, Ntege C, Meek SR, Abeku TA. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasit Vectors. 2016;9:100.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–7.
Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–13.
Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, et al. Genetic diversity and protective efficacy of the RTS, S/AS01 malaria vaccine. N Engl J Med. 2015;373:2025–37.
Espié E, Diene Sarr F, Diop F, Faye J, Richard V, Tall A, et al. Spatio-temporal variations in malaria incidence in children less than 10 years old, Health District of Sokone, Senegal, 2010–2013. PLoS ONE. 2015;10:e0137737.
Niang EHA, Touré A, Ngom EHM, Konaté L, Faye O, Diallo M, et al. Malaria transmission pattern in an area selected for clinical trials in the Sudanian Area of Senegal (West Africa). J Trop Med. 2013;2013:907375.
Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–37.
Snounou G, Zhu X, Siripoon N, Jarra W, Thaithong S, Brown KN, et al. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg. 1999;93:369–74.
Atroosh W, Al-Mekhlafi H, Mahdy M, Saif-Ali R, Al-Mekhlafi A, Surin J. Genetic diversity of Plasmodium falciparum isolates from Pahang, Malaysia based on MSP-1 and MSP-2 genes. Parasit Vectors. 2011;4:233.
Zwetyenga J, Rogier C, Tall A, Fontenille D, Snounou G, Trape J, et al. No influence of age on infection complexity and allelic distribution in Plasmodium falciparum infections in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Am J Trop Med Hyg. 1998;59:726–35.
Centers for Disease Control and Prevention. https://www.cdc.gov/dpdx/resources/pdf/benchAids/malaria/Parasitemia_and_LifeCycle.pdf. Accessed 24 July 2018.
Vafa M, Troye-Blomberg M, Anchang J, Garcia A, Migot-Nabias F. Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variants. Malar J. 2008;7:17.
Fontenille D, Lochouarn L, Diatta M, Sokhna C, Dia I, Diagne N, et al. Four years’ entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Trans R Soc Trop Med Hyg. 1997;91:647–52.
Färnert A, Lebbad M, Faraja L, Rooth I. Extensive dynamics of Plasmodium falciparum densities, stages and genotyping profiles. Malar J. 2008;7:241.
Konaté L, Zwetyenga J, Rogier C, Bischoff E, Fontenille D, Tall A, et al. Variation of Plasmodium falciparum msp1 block 2 and msp2 allele prevalence and of infection complexity in two neighbouring Senegalese villages with different transmission conditions. Trans R Soc Trop Med Hyg. 1999;93(Suppl 1):21–8.
Mohammed H, Mindaye T, Belayneh M, Kassa M, Assefa A, Tadesse M, et al. Genetic diversity of Plasmodium falciparum isolates based on msp-1 and msp-2 genes from Kolla-Shele area, Arbaminch Zuria District, southwest Ethiopia. Malar J. 2015;14:73.
Mayengue PI, Ndounga M, Malonga FV, Bitemo M, Ntoumi F. Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum isolates from Brazzaville, Republic of Congo. Malar J. 2011;10:276.
Peyerl-Hoffmann G, Jelinek T, Kilian A, Kabagambe G, Metzger WG, Von Sonnenburg F. Genetic diversity of Plasmodium falciparum and its relationship to parasite density in an area with different malaria endemicities in West Uganda. Trop Med Int Health. 2001;6:607–13.
Mayor A, Saute F, Aponte JJ, Almeda J, Gomez-Olive FX, Dgedge M, et al. Plasmodium falciparum multiple infections in Mozambique, its relation to other malariological indices and to prospective risk of malaria morbidity. Trop Med Int Health. 2003;8:3–11.
Beadle C, McElroy PD, Oster CN, Beier JC, Oloo AJ, Onyango FK, et al. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: implications for malaria vaccine trial design. J Infect Dis. 1995;172:1047–54.
McElroy PD, Beier JC, Oster CN, Beadle C, Sherwood JA, Oloo AJ, et al. Predicting outcome in malaria: correlation between rate of exposure to infected mosquitoes and level of Plasmodium falciparum parasitemia. Am J Trop Med Hyg. 1994;51:523–32.
Sonden K, Doumbo S, Hammar U, Vafa Homann M, Ongoiba A, Traore B, et al. Asymptomatic multiclonal Plasmodium falciparum infections carried through the dry season predict protection against subsequent clinical malaria. J Infect Dis. 2015;212:608–16.
Males S, Gaye O, Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis. 2008;46:516–22.
Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74.
Chen Q. The naturally acquired immunity in severe malaria and its implication for a PfEMP-1 based vaccine. Microbes Infect. 2007;9:777–83.
Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing, and efficacy: preventing and overcoming “vaccine resistant malaria”. Parasite Immunol. 2009;31:560–73.
Takala SL, Escalante AA, Branch OH, Kariuki S, Biswas S, Chaiyaroj SC, et al. Genetic diversity in the Block 2 region of the merozoite surface protein 1 (MSP-1) of Plasmodium falciparum: additional complexity and selection and convergence in fragment size polymorphism. Infect Genet Evol. 2006;6:417–24.
Authors’ contributions
ATB conceived and coordinated this study, contributed to the methodology, to the revision of the manuscript and approved the final version. BD contributed to the conception of the study, performed molecular biology studies and data analysis and drafted the manuscript. FD contributed to the conception of the study, performed field studies, participated in the revision of the manuscript and approved the final version. ID contributed to data analysis, participated in drafting the manuscript and approved the final version. CL conducted the statistical analysis, and approved the final version. YD contributed to the analysis, participated in drafting and revising the manuscript and approved the final version. JF performed field recruitments, lead the management of the database and approved the final version. MS contributed to the methodology, participated in revising the manuscript and approved the final version. RP contributed to drafting and revising the manuscript and approved the final version. MN participated in the design and the conducts of the molecular biology studies, contributed to revising the manuscript and approved the final version. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank all the stakeholders involved in this study, and particularly the IPD staff, the inhabitants of Toubacouta rural community, the district health teams, the health workers, the local authorities and the communities. The authors gratefully acknowledge the Pasteur Institute of Dakar and the National Health Ministry. The authors gratefully thank EDCTP for funding this study (Grant CB.07.41700.007).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the National Ethics Committee for Health Research of Senegal (CNERS). Written informed consent was obtained from parents or guardians of the children prior to recruitment. For those who were not literate, the protocol was translated into the local language, and consent was obtained in the presence of an independent witness.
Funding
This work was supported by Grant N° CB.2007.41700.007 (Capacity building to prepare West African sites for clinical trials on HIV, TB and malaria) from European and Developing Countries Clinical Trials Partnership (EDCTP, http://www.edctp.org/) in the framework of West African Network of Excellence for TB, AIDS, and Malaria (WANETAM) awarded to Aïssatou Toure-Balde. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Primers and the reaction conditions used to amplify msp-1 and msp-2 genes.
Additional file 2: Fig. S1.
Photographs of migration profiles of msp-1 and msp-2 allelic families in 1.5% agarose gel.
Additional file 3: Table S2.
Ranges of fragment sizes for msp-1 and msp-2 allelic families.
Additional file 4: Fig. S2.
Box plot of parasitaemia in samples from asymptomatic children infected with Plasmodium falciparum. The eight outliers removed prior to comparison of monoclonal and polyclonal infections are shown.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Diouf, B., Diop, F., Dieye, Y. et al. Association of high Plasmodium falciparum parasite densities with polyclonal microscopic infections in asymptomatic children from Toubacouta, Senegal. Malar J 18, 48 (2019). https://doi.org/10.1186/s12936-019-2684-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-019-2684-3