Abstract
Background
Onchocerciasis is a disease caused by infection with Onchocerca volvulus, which is transmitted to humans via the bite of several species of black fly, and is responsible for permanent blindness or vision loss, as well as severe skin disease. Predominantly endemic in parts of Africa and Yemen, preventive chemotherapy with mass drug administration of ivermectin is the primary intervention recommended for the elimination of its transmission.
Methods
A dataset of 18,116 geo-referenced prevalence survey datapoints was used to model annual 2000–2018 infection prevalence in Africa and Yemen. Using Bayesian model-based geostatistics, we generated spatially continuous estimates of all-age 2000–2018 onchocerciasis infection prevalence at the 5 × 5-km resolution as well as aggregations to the national level, along with corresponding estimates of the uncertainty in these predictions.
Results
As of 2018, the prevalence of onchocerciasis infection continues to be concentrated across central and western Africa, with the highest mean estimates at the national level in Ghana (12.2%, 95% uncertainty interval [UI] 5.0–22.7). Mean estimates exceed 5% infection prevalence at the national level for Cameroon, Central African Republic, Democratic Republic of the Congo (DRC), Guinea-Bissau, Sierra Leone, and South Sudan.
Conclusions
Our analysis suggests that onchocerciasis infection has declined over the last two decades throughout western and central Africa. Focal areas of Angola, Cameroon, the Democratic Republic of the Congo, Ethiopia, Ghana, Guinea, Mali, Nigeria, South Sudan, and Uganda continue to have mean microfiladermia prevalence estimates exceeding 25%. At and above this level, the continuation or initiation of mass drug administration with ivermectin is supported. If national programs aim to eliminate onchocerciasis infection, additional surveillance or supervision of areas of predicted high prevalence would be warranted to ensure sufficiently high coverage of program interventions.
Similar content being viewed by others
Background
Onchocerciasis is a disease caused by infection with the filarial nematode Onchocerca volvulus, which is transmitted to humans by the bite of the infected black fly (Simulium spp.). Over time, infection can cause permanent blindness or severe skin manifestations, including extreme and debilitating itching. Formerly endemic in focal areas of the Americas, the global distribution of onchocerciasis is now entirely concentrated in Africa and Yemen [1]. Interventions to control or interrupt transmission have been implemented since the mid-1970s, either through vector control (larviciding) or, since the late 1980s, using mass drug administration (MDA) with ivermectin. Preventive chemotherapy with MDA (in which all eligible individuals residing in endemic areas are offered ivermectin) is currently the primary intervention for the control of morbidity and elimination of transmission, largely delivered via the Community-Directed Treatment with Ivermectin (CDTI) strategy [2]. Over 1 billion ivermectin treatments have been donated to national onchocerciasis control programs, in addition to millions of treatments provided under the auspices of national lymphatic filariasis (LF) elimination programs.
Evidence from settings in Uganda [3] and Sudan [4], from the Onchocerciasis Elimination Program in the Americas [5, 6], and from modeling studies [7, 8] suggests there is a possibility that annual or more frequent MDA reaching at least 80% of the eligible population may halt transmission. The success of local elimination has led national programs, donors, implementing partners, the Mectizan Donation Program, and technical experts to consider the feasibility of onchocerciasis elimination in Africa [9]. Elimination is achieved as transmission is first suppressed through > 80% population coverage with annual MDA, and then ultimately interrupted as the reservoir of prevalent adult worms experiences mortality or infertility [10]. The duration of MDA required to eliminate transmission in Africa will vary by individual setting, with projections from simulation studies ranging from 10 to 25 years, depending on baseline prevalence and intensity of infection, population MDA coverage, and other local factors. Operational research is currently underway to refine guidelines for evaluating elimination programs, improve diagnostic test performance, and develop new therapeutics. Deployment of novel intervention strategies such as “test-and-not-treat” [11] is also being evaluated in areas co-endemic for O. volvulus and the filarial nematode Loa loa. MDA with ivermectin is contraindicated among individuals with loiasis due to a significant potential for severe neurological outcomes. The risk of severe adverse events may outweigh the benefits of ivermectin MDA in areas that are both endemic for loiasis and hypoendemic for onchocerciasis. Spatial prediction of onchocerciasis burden could benefit control programs by helping identify locations where alternative strategies may be needed for safe and effective elimination [12].
Achieving elimination of onchocerciasis transmission in Africa will require investment across the continent, from mapping surveys to identify and confirm areas requiring MDA to periodic monitoring of program impact in human and vector populations over at least a decade following initiation of interventions. According to the Expanded Special Project for the Elimination of Neglected Tropical Diseases (ESPEN), nearly 2000 districts may require some form of data collection to confirm eligibility for MDA with ivermectin [13]. Since local factors such as vector subspecies, human movement, and environmental conditions contribute to local variation in onchocerciasis prevalence, model-based geostatistics offers an opportunity to integrate the spatial and temporal relations in the existing evidence base to predict prevalence of onchocerciasis infection continuously, augmented with covariates to capture variation in the distribution of infection at finer spatial scales (see for example Cromwell et al. 2020) [14]. While these predictions are no substitute for primary data collection, they can be used to guide prioritization of areas to survey or targeted strengthening of MDA interventions. Such models have been used previously to estimate the pre-control [15] prevalence of skin snip positivity for the west African context as well as nodule prevalence for areas supported by the African Programme for Onchocerciasis Control [16]. To date, there are no contemporary geospatial estimates for the entire African continent or Yemen.
The objective of this analysis was to estimate the prevalence of onchocerciasis infection across the African continent and Yemen through time, quantifying the progress achieved in reducing onchocerciasis infection from 2000 to 2018, by accounting for ivermectin MDA implemented by national onchocerciasis control programs, as well as for the purpose of eliminating lymphatic filariasis as a public health problem. We also stratify these estimates of the number infected among areas identified to be high-risk for L. loa, as novel implementation strategies such as “test-and-not-treat” [11] will be required to achieve onchocerciasis elimination in these locations.
Methods
Data inputs
Data on the prevalence of onchocerciasis infection is largely collected by national onchocerciasis control and elimination programs as part of routine program monitoring. While methods for data collection vary by time and place, areas covered by the former Onchocerciasis Control Programme (OCP) in west Africa and the African Programme for Onchocerciasis Control (APOC), as well as onchocerciasis control programs supported by other partners, often identified areas (foci or districts) eligible for MDA or vector control by purposively sampling communities near known or suspected Simulium breeding sites. In OCP-supported areas, prevalence of onchocerciasis was estimated using skin snip biopsy (microscopy) to detect the presence of microfilariae; in APOC-areas, nodule (onchocercoma) palpation was used in the rapid epidemiological mapping for onchocerciasis (REMO) [17]. More recently, onchocerciasis programs have used Ov16 antibody testing by ELISA (enzyme-linked immunosorbent assay), in conjunction with entomological surveillance, as per WHO guidelines [10] to demonstrate elimination of transmission, and the use of rapid diagnostic tests is being evaluated for programmatic use. We compiled an analytical dataset of onchocerciasis infection prevalence from the following sources: a systematic review of literature in which data collected between 1988 and the present were included in the analysis (Additional file 1: Fig. S2 and Table S3); the ESPEN online portal [18]; and personal communication for data collected under the OCP [15] from its former Director, BA Boatin, PhD (personal communication, January 2019). Data were reviewed and geo-referenced either to point locations (i.e., a community) or polygons (i.e., areal data attributed to a focus or district). In this analysis, we included data for which nodule palpation or skin snip biopsy was reported. A total of 17,896 point-referenced and 220 polygon-referenced inputs were included in the analysis, with 14,314 total inputs initially reported as nodule prevalence and 3,802 as skin snip biopsy. Further details on the dataset are presented in Additional file 1: Section 3.
Geospatial covariates
In order to develop a predictive model of onchocerciasis prevalence that was generalizable to under-surveyed locations and years, we sought to include a suite of environmental covariates that may be associated with the presence or intensity of O. volvulus transmission (Additional file 1: Table S5 and Fig. S4). We compiled covariates that collectively provide a broad characterization of local ecological conditions, including precipitation, temperature, aridity, orographic slope, vegetation, soil characteristics, distance to rivers, and maximum river width. Human population density was also included to accommodate a possible association with urbanicity. Cumulative years of any MDA with ivermectin for onchocerciasis or lymphatic filariasis (as a single covariate) were included. Finally, we included outputs from a recent model of onchocerciasis environmental suitability (Additional file 1: Fig. S8; Cromwell and colleagues [19]) to incorporate environmental effects calibrated by onchocerciasis presence data. Raw covariate raster surfaces were resampled to a consistent 5 × 5-km grid-cell resolution (see Additional file 1: Section 4.1). Time-varying covariates (e.g., climatic variables and interventions) were associated with their corresponding model years, except when specific years of data were unavailable for a given covariate, in which case the nearest available year of data was used (covariate temporal coverage is listed in Additional file 1: Table S5). Analysis of variance inflation factors [20] (VIF, with a VIF threshold of 3.0) was used to exclude collinear covariates (Additional file 1: Section 4.3). Model reliability is affected by the overlap between covariate values in training and prediction datasets (see Additional file 1: Fig. S7). Predictions in regions with covariate values falling outside the range of training values may be prone to extrapolation errors and should be considered with special caution. Such areas include the Sahel and Sahara, Yemen, Kenya, Somalia, eastern Ethiopia, and southern Angola.
Age and diagnostic adjustment
In order to derive global estimates of onchocerciasis infection using data reported across different age ranges and diagnostic tests, we used age and diagnostic models to adjust (“crosswalk”) input data prior to the main modeling analysis, yielding estimates of both-sex, all-age (0–94 years) microfiladermia prevalence as measured by skin snip microscopy. To develop models to adjust age-specific data to all-age prevalence or to adjust nodule prevalence data to skin snip microscopy, we identified peer-reviewed published surveys that reported skin snip or nodule prevalence, or both, in multiple age groups within the same study populations, from countries included in the geospatial modeling region (Additional file 1: Table S6). Diagnostic effects and non-linear prevalence-by-age relationships were estimated simultaneously by maximum likelihood optimization of a logistic regression model, using separate basis splines on age for each diagnostic test (skin snips and nodules), an indicator variable for skin snip surveys, and study population-level fixed effects. Scaling factors were then estimated for each observation in the full geospatial modeling dataset, by fixing model coefficients to the mean estimates derived from the training set and optimizing the study population-level effects via maximum likelihood. Reported prevalence values were adjusted by applying these scaling factors to the inferred (age and diagnostic models) all-age prevalence curves for the reported diagnostic type, yielding estimates of all-age skin snip prevalence. These crosswalked prevalence values were used as outcome data in the geospatial model. Further details about the diagnostic and age adjustment methodology and results are provided in Additional file 1: Section 5.1.
Geostatistical analysis
A Bayesian geostatistical model [21, 22] was fit for the group of African countries (plus Yemen) known or suspected to include locations endemic for onchocerciasis as defined by ESPEN. Justification of the geographical restrictions used to establish the modeling region is presented in Additional file 1: Section 3 and Table S2. While we were primarily concerned with prevalence estimates for the time period 2000–2018, we fit the model using data from 1988 to 2018 in order to incorporate data from pre-2000 OCP and APOC surveys and thereby improve estimates in countries covered by those programs. Reporting of results focuses on estimates for 2000–2018.
The full onchocerciasis prevalence model was a spatial generalized linear mixed effects model using a binomial likelihood and minimally informative priors (Additional file 1: Section 5.3 and Table S7). The model was estimated by integrated nested Laplace approximation (INLA) [23] within the R package R-INLA [24]. Covariates were included as fixed effects, except that estimates from the onchocerciasis suitability model were incorporated using a second-order random walk model to accommodate non-linearity. The model included country-level random effects to account for variation in national onchocerciasis burdens and control programs, and a nugget variance term to accommodate fine-spatial scale and sampling variation. A spatial Gaussian process was used to model residual spatial variation, using stochastic partial differential equations (SPDE) [25] and a Matérn spatial covariance function. Predictions were generated at a 5 × 5-km spatial resolution, with 1,000 samples drawn from the joint posterior distribution. Predictions were summarized using the means and 95% uncertainty intervals (UI; 2·5th and 97·5th percentiles) from the 1,000 draws of prevalence.
Aggregate estimates of onchocerciasis prevalence were calculated using population-weighted means of grid-cell-level prevalence, with weighting by WorldPop [26] grid-cell-level modeled population estimates calibrated to match Global Burden of Disease population estimates at national or administrative subunit level 1 (where available). Estimates were aggregated across 5 × 5-km cells within administrative boundaries at national and administrative levels 1 and 2, using updated administrative shapefiles originally supplied by GADM (Global Administrative Areas) [27]. We first masked all final model outputs for which land cover was classified as “barren or sparsely vegetated” by Moderate Resolution Imaging Spectroradiometer satellite data for 2015 [28], as well as areas in which total population density in 2015 was less than ten individuals per 1 × 1-km grid cell by WorldPop population estimates. Estimates from such locations (e.g., the southern Sahara Desert) are considered less reliable due to sparse prevalence data sampling and extreme covariate values. We retained input data from such areas in the model because they are still informative about the spatial distribution of onchocerciasis prevalence and its relationship with model covariates.
Five-fold cross-validation was used for out-of-sample model validation. The geostatistical model was run five times, each time holding out data from one spatially stratified fold and generating predictions for the held-out data. A suite of measures of out-of-sample performance were examined, namely bias, mean absolute error, root mean square error, 95% prediction interval data coverage, and correlations of observed to predicted values. The data processing and modeling workflows for this study are outlined in Additional file 1: Fig. S1. All statistical analysis was performed using statistical software R v.3.5.1.
Results
As of 2018, the prevalence of onchocerciasis infection continues to be concentrated across central Africa, with the highest prevalence areas in focal areas of the Democratic Republic of the Congo (DRC), Ghana, Nigeria, Cameroon, and South Sudan, based on mean predictions at the 5 × 5-km resolution. Mean prevalence predictions were also above 10% in focal areas of several additional countries, including Angola, Ethiopia, Gabon, Nigeria, and the Republic of the Congo. Mean prevalence at the national level was highest in Ghana (12.2%, 95% uncertainty interval [UI] 5.0–22.7) and Equatorial Guinea (9.7%, 8.0–11.7), with mean estimates also exceeding 5% infection prevalence at the national level for Cameroon, Central African Republic, DRC, Guinea-Bissau, Sierra Leone, and South Sudan.
Our model estimates should be considered in the context of model performance (Additional file 1: Fig. S11 and Table S9). Overall out-of-sample bias was low, with a mean error of 0.003 (0.3% in prevalence space) across all model years (1988–2018). The variation over time and space of mean error and other performance metrics, including mean absolute error (overall value: 0.111, or 11.1%), RMSE (overall value: 0.168, or 16.8%), and correlation (overall value: 0.706), and the sometimes wide uncertainty intervals of predictions (both in- and out-of-sample) reflect in part limited data on onchocerciasis infection prevalence across the time series for many locations. In other areas where data are unavailable, such as southern Kenya or the border between Sudan and South Sudan, covariate patterns are under-represented in the input data and our predictions should be interpreted in conjunction with other programmatic data sources.
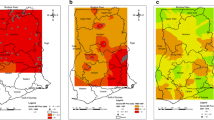
As illustrated in Fig. 1, while the analysis shows large declines overall in the prevalence of onchocerciasis from 2000 to 2018, much of central Africa would continue to warrant MDA with ivermectin (among districts for which Loa loa is non-endemic) or consideration for “test-and-not-treat” implementation in areas where MDA might be broadly contraindicated due to high loiasis burden. In central Africa, much of the high infection prevalence is, in part, among areas ineligible for ivermectin due to loiasis burden, or areas of greater insecurity or inaccessibility. The model predicts low (under 1%) infection prevalence for nearly all areas in northern and central Burkina Faso, central and eastern Niger, northern Guinea, northern Côte d’Ivoire, eastern Ethiopia, Kenya, and much of Tanzania. The uncertainty (Fig. 2) of these predictions is high, particularly for estimates from 2000 to 2005, at both the fine-spatial scale (5 × 5-km resolution), as well as national and subnational-level predictions. Detailed model results, including uncertainty results and temporal trends, are also available for scrutiny in an interactive visualization tool at https://vizhub.healthdata.org/lbd/oncho.
Prevalence of O. volvulus microfiladermia in Africa and Yemen at the 5 × 5-km level. Mean predictions of O. volvulus microfiladermia prevalence (all-age, both sexes) from the Bayesian geostatistical model, as measured by skin snip biopsies and crosswalked nodule palpation surveys. Hatch-marks indicate countries for which estimates were not produced; grey areas are masked based on sparsely populated areas (fewer than ten people per 1 × 1-km grid cell) and barren landscape classification. Data can be viewed on an interactive visualization tool at https://vizhub.healthdata.org/lbd/oncho
Africa and Yemen O. volvulus microfiladermia model uncertainty at the 5 x 5-km level. Mean and absolute uncertainty (measured as the range, or difference between, the upper and lower 95% UI) in O. volvulus microfiladermia prevalence estimates (all-age, both sexes) in Africa and Yemen. Hatch-marks indicate countries for which estimates were not produced; grey areas are masked based on sparsely populated areas (fewer than ten people per 1 × 1-km grid cell) and barren landscape classification. Quantile breakpoints for plotted categories are 0.001 (25th percentile), 0.009 (50th percentile), and 0.048 (75th percentile) for mean prevalence, and 0.009, 0.054, and 0.258 for range. Data can be viewed on an interactive visualization tool at https://vizhub.healthdata.org/lbd/oncho.
For the period 2000–2018, most national onchocerciasis programs aimed to control morbidity, not eliminate transmission. As such, high-burden areas typically received interventions with CDTi. In Fig. 3, we present the median, minimum, and maximum second-order administrative unit-level prevalence estimates for 2000 and 2018. This comparison illustrates the reductions in infection prevalence achieved, narrowing the gap between high-burden and low-burden districts. Such reductions are most notable in Cameroon, Ghana, and Sierra Leone. In areas of Uganda and Sudan known to have achieved elimination of transmission, our model predictions are consistent with observed data. In the Abu Hamed focus (Sudan), our results are consistent with elimination targets being met by 2007 [29]. In Uganda, our model results are consistent with program progress in the 15 foci for which MDA has ceased [30].
Distribution of onchocerciasis prevalence within and among countries at administrative level 2 (2000 and 2018). The median (central points) and lowest–highest (bars) mean prevalence estimates at administrative level 2 are shown for each country in the modeling region, for 2000 and 2018. Countries are ordered by increasing median administrative level 2 prevalence in 2018
Discussion
This analysis quantifies the impact of nearly two decades of national onchocerciasis control and elimination program activities. While there have been substantial reductions in the overall prevalence of infection that should be celebrated, like so many health metrics [22, 31], this has not been achieved equally throughout the continent. Prior geospatial analyses of onchocerciasis prevalence [15, 16] have been limited to regional estimates for west Africa and the APOC areas (central and eastern Africa). Our analysis is qualitatively very similar to these previous estimates of high nodule prevalence, showing particularly high burden in the DRC [16]. This is expected, as the relationship between nodule prevalence and skin snip positivity has better agreement at higher levels of prevalence [32]. Although our model includes data collected throughout the continent for both skin snip and nodule prevalence (with nodule prevalence adjusted to represent prevalence of infection measured via skin snip), our model predictions for 2011 (available via the online visualization) are qualitatively similar to the estimates published by Zouré and colleagues [16]. Our analysis extends prediction to include areas masked from that analysis based on expert opinion on suitability, as we intend these model results to be useful for decision-making by national programs for locations under consideration for onchocerciasis elimination mapping, in conjunction with the best local evidence.
We envision three specific use cases for this analysis. First, overall onchocerciasis burden estimates should reflect the implementation of nearly 20 years of MDA for both onchocerciasis and lymphatic filariasis programs. This geospatial model will be used in future updates to the Global Burden of Disease Study [33] to adjust estimates of contemporary morbidity due to onchocerciasis. Second, as national onchocerciasis programs are currently in the process of consolidating historical evidence, plans for elimination of transmission including additional mapping surveys and MDA will require such evidence to be aggregated for decision-making and prioritization. In light of limited resources, programs may wish to consider using these maps, alongside other data sources, to identify priority areas or evaluate potential low-endemicity areas for program eligibility. In the case of areas potentially co-endemic for LF and onchocerciasis, these results may enable program managers to prioritize timing of post-MDA surveillance for both pathogens, rather than ceasing MDA for LF while onchocerciasis infection may still be prevalent. Third, in areas co-endemic for Loa loa, this analysis may serve to quantify program targets for novel MDA implementation strategies.
By producing estimates for all known endemic countries in Africa and Yemen, this analysis is comprehensive in scope for the locations currently under consideration for the elimination of transmission. We conducted a systematic literature review to identify historical prevalence data and included publicly available prevalence data provided by national programs via the ESPEN online data portal. Additional data from the former OCP areas were also included, substantially strengthening our predictions for west Africa. We also developed models for age and diagnostic adjustment in order to leverage data reported across multiple age categories and reconcile the two dominant diagnostic methods employed in program monitoring. Our approach using model-based geostatistics enables us to predict prevalence while accounting for a broad range of covariates associated with onchocerciasis and other neglected tropical diseases optimized for prediction, to be of maximal utility to programs. We developed a geospatial covariate of MDA with ivermectin to account for the impact of both onchocerciasis and lymphatic filariasis programs.
The analysis has several limitations we wish to acknowledge. First, it is possible that covariate patterns do not adequately capture the ecological niche for Simulium in all settings, particularly given the flight range of the vector exceeds 5 km [34]. Combined with human movement, it is possible that the locations for which communities test positive may not directly correlate with where individuals are infected. Simulium density data are not widely available; therefore, we are unable to include measures of the vector as a covariate. These model results could be compared against more detailed remote sensing analyses for specific locations; however, the fine spatial scale of those approaches would be computationally infeasible at the continental scale. Further, we do not have complete enumeration of breeding sites, and so the analysis assumes other covariates represent ecological conditions that might be suitable for transmission and are a sufficient proxy for exposure to both the vector and O. volvulus. There may be settings where seasonal rivers enable establishment of viable breeding sites, and future analysis could consider more detailed hydrological data sources. We excluded serological prevalence data inputs, as the relationship between antibody positivity and population-level infection prevalence was unstable, and variability exists in the performance of specific antibody-based diagnostic methods or protocols [35]. Less than 1% of the total input data we obtained was measured using serological tests (from a total of seven countries), and exclusion of these data from preliminary models resulted in negligible differences in the results. Prevalence of microfilariae measured by skin snip biopsy is also subject to limitations as sensitivity is lower in low-prevalence settings. Future work should consider the possibility of false negatives, particularly in pre-control data inputs. Future work is needed to incorporate Ov16 serological tests into the modeling framework, as more programs will use this diagnostic for end of program surveillance, as well as baseline mapping of districts for which contemporary evidence is unavailable. While our model does include MDA as a covariate, we did not use reported coverage (i.e., percentage of the population that received treatment). Data on reported coverage by district are unavailable for all implementation units across the time series, and reported coverage has been demonstrated to be biased [36]. We did not include explicit temporal terms in the model because extensive time series exist for relatively few locations (see the spatial and temporal distribution of available data in Additional file 1: Fig. S3), and exploratory spatiotemporal models yielded unrealistically erratic temporal trends. Allowing temporal changes in prevalence to be driven by the covariates produced more tenable trends, but the resulting model may be insensitive to particularly rapid prevalence changes in some localities. Finally, as prevalence data were collected for the purposes of program monitoring, there is likely heterogeneity in the quality of field-based data collection that we are unable to account for in this model.
Conclusions
The feasibility of elimination of onchocerciasis transmission throughout Africa is currently under consideration by national programs, implementing partners, donors, and drug-donating pharmaceutical companies. While areas of high prevalence remain, our analysis shows that programs have been extremely successful in reducing prevalence across high-endemicity locations. We present the first time series estimates of infection prevalence to quantify the gains currently achieved by control and elimination interventions to assist with prioritization and program planning. It is for decision-makers at all levels to decide if elimination is a feasible goal.
Availability of data and materials
Detailed model results, including uncertainty results, are available through an interactive visualization tool at https://vizhub.healthdata.org/lbd/oncho. The data sources and code used to generate these estimates, as well as tables of mean estimates and uncertainty intervals, are publicly available online at the Global Health Data Exchange (GHDx; http://ghdx.healthdata.org/).
Abbreviations
- APOC:
-
African Programme for Onchocerciasis Control
- CDTI:
-
Community-Directed Treatment with Ivermectin
- DRC:
-
Democratic Republic of the Congo
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESPEN:
-
Expanded Special Project for the Elimination of Neglected Tropical Diseases
- GADM:
-
Global Administrative Areas
- INLA:
-
Integrated nested Laplace approximation
- LF:
-
Lymphatic filariasis
- MDA:
-
Mass drug administration
- OCP:
-
Onchocerciasis Control Programme
- REMO:
-
Rapid epidemiological mapping for onchocerciasis
- SPDE:
-
Stochastic partial differential equations
- VIF:
-
Variance Inflation Factor
References
World Health Organization. Progress report on the elimination of human onchocerciasis, 2016–2017. Wkly Epidemiol Rec. 2017;45:681–694.
Lawrence J, Sodahlon YK, Ogoussan KT, Hopkins AD. Growth, challenges, and solutions over 25 years of mectizan and the impact on onchocerciasis control. PLoS Negl Trop Dis. 2015;9(5):e0003507.
Lakwo TL, Garms R, Rubaale T, Katabarwa M, Walsh F, Habomugisha P, et al. The disappearance of onchocerciasis from the Itwara focus, western Uganda after elimination of the vector Simulium neavei and 19 years of annual ivermectin treatments. Acta Trop. 2013;126(3):218–21.
Zarroug IMA, Hashim K, ElMubark WA, Shumo ZAI, Salih KAM, ElNojomi NAA, et al. The first confirmed elimination of an onchocerciasis focus in Africa: Abu Hamed, Sudan. Am J Trop Med Hyg. 2016;95(5):1037–40.
Sauerbrey M, Rakers LJ, Richards FO. Progress toward elimination of onchocerciasis in the Americas. Int. Health. 2018;10(suppl_1):i71–8.
Gonzalez RJ, Cruz-Ortiz N, Rizzo N, Richards J, Zea-Flores G, Domínguez A, et al. Successful interruption of transmission of Onchocerca volvulus in the Escuintla-Guatemala focus, Guatemala. PLoS Negl Trop Dis. 2009;3(3):e404.
Coffeng LE, Stolk WA, Hoerauf A, Habbema D, Bakker R, Hopkins AD, et al. Elimination of African onchocerciasis: modeling the impact of increasing the frequency of ivermectin mass treatment. PLoS One. 2014;9(12):e115886.
Verver S, Walker M, Kim YE, Fobi G, Tekle AH, Zouré HGM, et al. How can onchocerciasis elimination in Africa be accelerated? Modeling the impact of increased ivermectin treatment frequency and complementary vector control. Clin Infect Dis. 2018;66(suppl_4):S267–74.
Lawrence J, Sodahlon YK. Onchocerciasis: the beginning of the end. Int. Health. 2018;10(suppl_1):i1–2.
World Health Organization. Onchocerciasis: guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis. Geneva: World Health Organization. Accessed 20 May 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/204180/9789241510011_eng.pdf;jsessionid=56D4BE77F31B8BA09A80672C34DBDD10?sequence=1.
Kamgno J, Pion SD, Chesnais CB, Bakalar MH, D’Ambrosio MV, Mackenzie CD, et al. A test-and-not-treat strategy for onchocerciasis in Loa loa–endemic areas. N Engl J Med. 2017;377(21):2044–52.
Vinkeles Melchers NVS, Coffeng LE, Boussinesq M, Pedrique B, Pion SDS, Tekle AH, et al. Projected number of people with onchocerciasis–loiasis coinfection in Africa, 1995 to 2025. Clin Infect Dis. 2020;70(11):2281–9.
ESPEN Portal. Accessed 20 May 2020. Available from: https://admin.espen.afro.who.int/.
Cromwell EA, Schmidt CA, Kwong KT, Pigott DM, Mupfasoni D, Biswas G, et al. The global distribution of lymphatic filariasis, 2000–18: a geospatial analysis. Lancet Glob Health. 2020;8(9):e1186–94.
O’Hanlon SJ, Slater HC, Cheke RA, Boatin BA, Coffeng LE, Pion SDS, et al. Model-based geostatistical mapping of the prevalence of Onchocerca volvulus in West Africa. PLoS Negl Trop Dis. 2016;10(1):e0004328.
Zouré HG, Noma M, Tekle AH, Amazigo UV, Diggle PJ, Giorgi E, et al. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis control: (2) pre-control endemicity levels and estimated number infected. Parasit Vectors. 2014;7(1):326.
Noma M, Nwoke BEB, Nutall I, Tambala PA, Enyong P, Namsenmo A, et al. Rapid epidemiological mapping of onchocerciasis (REMO): its application by the African Programme for Onchocerciasis control (APOC). Ann Trop Med Parasitol. 2002;96(Suppl 1):S29–39.
World Health Organization: Regional Office for Africa. Onchocerciasis. ESPEN. 2019. Accessed 14 May 2019. Available from: http://espen.afro.who.int/diseases/onchocerciasis.
Cromwell EA, Osborne JCP, Unnasch TR, Basáñez MG, Gass KM, Barbre KA, et al. Predicting the environmental suitability for onchocerciasis in Africa as an aid to elimination planning. PLoS Negl Trop Dis. 2021;15(7):e0008824.
Faraway JJ. Linear models with R. Boca Raton: CRC Press; 2005.
Golding N, Burstein R, Longbottom J, Browne AJ, Fullman N, Osgood-Zimmerman A, et al. Mapping under-5 and neonatal mortality in Africa, 2000–15: a baseline analysis for the sustainable development goals. Lancet. 2017;390(10108):2171–82.
Osgood-Zimmerman A, Millear AI, Stubbs RW, Shields C, Pickering BV, Earl L, et al. Mapping child growth failure in Africa between 2000 and 2015. Nature. 2018;555(7694):41–7.
Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Ser B Stat Methodol. 2009;71(2):319–92.
Lindgren F, Rue H. Bayesian spatial Modelling with R- INLA. J Stat Softw. 2015;63(19):1–25.
Lindgren F, Rue H, Lindström J. An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. Stat Methodol Ser B. 2011;73(4):423–98.
WorldPop. WorldPop dataset. 2017. Accessed 24 July 2017. Available from: http://www.worldpop.org.uk/data/get_data/.
Global Administrative Areas (GADM). GADM database of global administrative areas. 2019. Accessed 11 June 2019. Available from: https://gadm.org.
Land Processes Distributed Active Archive Center (LPDAAC). Combined MODIS 5.1. MCD12Q1 | LP DAAC: NASA Land Data Products and Services. 2017. Accessed 1 June 2017. Available from: https://lpdaac.usgs.gov/dataset_discovery/modis/modis_products_table/mcd12q1.
Higazi TB, Zarroug IMA, Mohamed HA, ElMubark WA, Deran TCM, Aziz N, et al. Interruption of Onchocerca volvulus transmission in the Abu Hamed focus, Sudan. Am J Trop Med Hyg. 2013;89(1):51–7.
Katabarwa MN, Lakwo T, Habomugisha P, Unnasch TR, Garms R, Hudson-Davis L, et al. After 70 years of fighting an age-old scourge, onchocerciasis in Uganda, the end is in sight. Int. Health. 2018;10(suppl_1):i79–88.
Burstein R, Henry NJ, Collison ML, Marczak LB, Sligar A, Watson S, et al. Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019;574(7778):353–8.
Coffeng LE, Pion SDS, O’Hanlon S, Cousens S, Abiose AO, Fischer PU, et al. Onchocerciasis: the pre-control association between prevalence of palpable nodules and skin microfilariae. PLoS Negl Trop Dis. 2013;7(4):e2168.
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–858.
Thompson BH. Studies on the flight range and dispersal of Simulium damnosum (Diptera: Simuliidae) in the rain-forest of Cameroon. Ann Trop Med Parasitol. 1976;70(3):343–54.
Unnasch TR, Golden A, Cama V, Cantey PT. Diagnostics for onchocerciasis in the era of elimination. Int Health. 2018;10(suppl_1):i20–6.
Worrell C, Mathieu E. Drug coverage surveys for neglected tropical diseases: 10 years of field experience. Am J Trop Med Hyg. 2012;87(2):216–22.
Acknowledgements
Data accessed via the ESPEN portal belong to the Ministries of Health and we would like to acknowledge the national onchocerciasis control and elimination programs for making these data available. F Carvalho FCT acknowledges Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences - UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy - i4HB; FCT/MCTES (Ministério da Ciência, Tecnologia e Ensino Superior) through the project UIDB/50006/2020M Ausloos, A Pana and C Herteliu are partially supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project number PN-III-P4-ID-PCCF-2016-0084. C Herteliu and A Pana are partially supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project number PN-III-P2-2.1-SOL-2020-2-0351. A M Samy acknowledges the support from the Egyptian Fulbright Mission program and Ain Shams University. A I Ribeiro was supported by National Funds through FCT, under the program of Stimulus of Scientific Employment “Individual Support” within the contract CEECIND/02386/2018. A Sheikh acknowledges the support of Health Data Research UK. M N Khan acknowledges the support of Jatiya Kabi Kazi Nazrul Islam University, Mymensingh, Bangladesh. B Unnikrishnan acknowledges Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal. K Deribe is supported by the Wellcome Trust [grant number 201900/Z/16/Z] as part of his International Intermediate Fellowship. O Adetokunboh acknowledges the South African Department of Science and Innovation, and National Research Foundation. Y J Kim acknowledges support by the Research Management Centre, Xiamen University Malaysia [No. XMUMRF/2020-C6/ITCM/0004]. T Bärnighausen was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award, funded by the German Federal Ministry of Education and Research. W Stolk gratefully acknowledges funding from the NTD Modelling Consortium by the Bill & Melinda Gates Foundation (grant number OPP1184344).
The members of the LBD 2019 Neglected Tropical Diseases Collaborators are the authors of this paper: Chris A Schmidt,1 Elizabeth A Cromwell,1,2 Elex Hill,1 Katie M Donkers,1 Megan F Schipp,1 Kimberly B Johnson,1 David M Pigott,1,2 Jaffar Abbas,3 Victor Adekanmbi,4 Olatunji O Adetokunboh,5,6 Muktar Beshir Ahmed,7,8 Fahad Mashhour Alanezi,9 Turki M Alanzi,10 Vahid Alipour,11,12 Catalina Liliana Andrei,13 Prof Tudorel Andrei,14 Davood Anvari,15,16 Seth Christopher Yaw Appiah,17,18 Muhammad Aqeel,19,19 Jalal Arabloo,11 Prof Mohammad Asghari Jafarabadi,20,21 Prof Marcel Ausloos,22,14 Atif Amin Baig,23 Prof Maciej Banach,24,25 Prof Till Winfried Bärnighausen,26,27 Krittika Bhattacharyya,28,29 Prof Zulfiqar A Bhutta,30,31 Ali Bijani,32 Oliver J Brady,33 Nicola Luigi Bragazzi,34 Zahid A Butt,35,36 Prof Felix Carvalho,37 Vijay Kumar Chattu,38,39 Saad M A Dahlawi,40 Giovanni Damiani,41,42 Feleke Mekonnen Demeke,43 Kebede Deribe,44,45 Prof Samath Dhamminda Dharmaratne,46,2,1 Prof Daniel Diaz,47,48 Alireza Didarloo,49 Lucas Earl,1 Prof Maysaa El Sayed Zaki,50 Prof Maha El Tantawi,51 Nazir Fattahi,52 Prof Eduarda Fernandes,53 Nataliya A Foigt,54 Masoud Foroutan,55,56 Richard Charles Franklin,57 Prof Yuming Guo,58,59 Arvin Haj-Mirzaian,60,61 Prof Samer Hamidi,62 Hadi Hassankhani,63,64 Prof Claudiu Herteliu,14,65 Prof Tarig B Higazi,66 Prof Mostafa Hosseini,67,68 Mehdi Hosseinzadeh,69,70 Prof Mowafa Househ,71 Olayinka Stephen Ilesanmi,72,73 Irena M Ilic,74 Prof Milena D Ilic,75 Seyed Sina Naghibi Irvani,64 Ravi Prakash Jha,76,77 John S Ji,78 Prof Jost B Jonas,79,80 Jacek Jerzy Jozwiak,81 Leila R Kalankesh,82 Naser Kamyari,83 Prof Behzad Karami Matin,52 Salah Eddin Karimi,84 Gbenga A Kayode,85,86 Ali Kazemi Karyani,52 Ejaz Ahmad Khan,87 Md Nuruzzaman Khan,88 Khaled Khatab,89,90 Mona M Khater,91 Neda Kianipour,92 Yun Jin Kim,93 Soewarta Kosen,94 Dian Kusuma,95,96 Prof Carlo La Vecchia,97 Prof Van Charles Lansingh,98,99 Paul H Lee,100 Shanshan Li,101 Shokofeh Maleki,102 Mohammad Ali Mansournia,67 Francisco Rogerlândio Martins-Melo,103 Colm McAlinden,104 Walter Mendoza,105 Tomislav Mestrovic,106,107 Masoud Moghadaszadeh,108,109 Abdollah Mohammadian-Hafshejani,110 Seyyede Momeneh Mohammadi,111 Shafiu Mohammed,112,113 Rahmatollah Moradzadeh,114 Paula Moraga,115 Mehdi Naderi,102 Ahamarshan Jayaraman Nagarajan,116,117 Ionut Negoi,118,119 Cuong Tat Nguyen,120 Huong Lan Thi Nguyen,120 Prof Bogdan Oancea,121 Andrew T Olagunju,122,123 Ahmed Omar Bali,124 Prof Obinna E Onwujekwe,125 Adrian Pana,14,126 Prof Vafa Rahimi-Movaghar,127 Kiana Ramezanzadeh,60 David Laith Rawaf,128,129 Prof Salman Rawaf,130,131 Reza Rawassizadeh,132 Aziz Rezapour,11 Ana Isabel Ribeiro,133 Abdallah M Samy,134 Masood Ali Shaikh,135 Kiomars Sharafi,52 Prof Aziz Sheikh,136,137 Prof Jasvinder A Singh,138,139 Eirini Skiadaresi,140 Shahin Soltani,52 Wilma A Stolk,141 Mu'awiyyah Babale Sufiyan,142 Alan J Thomson,143 Bach Xuan Tran,144 Khanh Bao Tran,145,146 Prof Bhaskaran Unnikrishnan,147 Prof Francesco S Violante,148,149 Giang Thu Vu,150 Tomohide Yamada,151 Prof Sanni Yaya,152,153 Prof Paul Yip,154,155 Naohiro Yonemoto,156,157 Prof Chuanhua Yu,158 Yong Yu,159 Maryam Zamanian,114 Yunquan Zhang,160,161 Zhi-Jiang Zhang,162 Arash Ziapour,163 Prof Simon I Hay.1,2
1Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA; 2Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA; 3Antai College of Economics, Shanghai Jiao Tong University, Shanghai, China; 4Department of Population Medicine, Cardiff University, Cardiff, UK; 5DSI-NRF Centre of Excellence for Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Stellenbosch, South Africa; 6Division of Epidemiology & Biostatistics, Stellenbosch University, Cape Town, South Africa; 7Department of Epidemiology, Jimma University, Jimma, Ethiopia; 8Australian Center for Precision Health, University of South Australia, Adelaide, SA, Australia; 9Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 10Health Information Management and Technology Department, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 11Health Management and Economics Research Center, Iran University of Medical Sciences, Tehran, Iran; 12Department of Health Economics, Iran University of Medical Sciences, Tehran, Iran; 13Cardiology Department, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; 14Department of Statistics and Econometrics, Bucharest University of Economic Studies, Bucharest, Romania; 15Department of Parasitology, Mazandaran University of Medical Sciences, Sari, Iran; 16Department of Parasitology, Iranshahr University of Medical Sciences, Iranshahr, Iran; 17Department of Sociology and Social Work, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; 18Center for International Health, Ludwig Maximilians University, Munich, Germany; 19Department of Psychology, Foundation University Islamabad, Rawalpandi, Pakistan; 20Department of Biostatistics and Epidemiology, Tabriz University of Medical Sciences, Tabriz, Iran; 21Department of Biostatistics and Epidemiology, Zanjan University of Medical Sciences, Zanjan, Iran; 22School of Business, University of Leicester, Leicester, UK; 23Unit of Biochemistry, Universiti Sultan Zainal Abidin (Sultan Zainal Abidin University), Kuala Terengganu, Malaysia; 24Department of Hypertension, Medical University of Lodz, Lodz, Poland; 25Polish Mothers' Memorial Hospital Research Institute, Lodz, Poland; 26Heidelberg Institute of Global Health (HIGH), Heidelberg University, Heidelberg, Germany; 27T.H. Chan School of Public Health, Harvard University, Boston, MA, USA; 28Department of Statistical and Computational Genomics, National Institute of Biomedical Genomics, Kalyani, India; 29Department of Statistics, University of Calcutta, Kolkata, India; 30Centre for Global Child Health, University of Toronto, Toronto, ON, Canada; 31Centre of Excellence in Women & Child Health, Aga Khan University, Karachi, Pakistan; 32Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran; 33Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK; 34University of Genoa, Genoa, Italy; 35School of Public Health and Health Systems, University of Waterloo, Waterloo, ON, Canada; 36Al Shifa School of Public Health, Al Shifa Trust Eye Hospital, Rawalpindi, Pakistan; 37Research Unit on Applied Molecular Biosciences (UCIBIO), University of Porto, Porto, Portugal; 38Department of Community Medicine, Datta Meghe Institute of Medical Sciences, Sawangi, India; 39Saveetha Medical College, Saveetha University, Chennai, India; 40Environmental Health Department, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 41IRCCS Istituto Ortopedico Galeazzi (Galeazzi Orthopedic Institute IRCCS), University of Milan, Milan, Italy; 42Department of Dermatology, Case Western Reserve University, Cleveland, OH, USA; 43Department of Medical Laboratory Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 44Wellcome Trust Brighton and Sussex Centre for Global Health Research, Brighton and Sussex Medical School, Brighton, UK; 45School of Public Health, Addis Ababa University, Addis Ababa, Ethiopia; 46Department of Community Medicine, University of Peradeniya, Peradeniya, Sri Lanka; 47Center of Complexity Sciences, National Autonomous University of Mexico, Mexico City, Mexico; 48Faculty of Veterinary Medicine and Zootechnics, Autonomous University of Sinaloa, Culiacán Rosales, Mexico; 49Department of Community Medicine and Public Health, Urmia University of Medical Science, Urmia, Iran; 50Reference Laboratory of Egyptian Universities-Cairo, Ministry of Higher Education and Scientific Research, Cairo, Egypt; 51Pediatric Dentistry and Dental Public Health Department, Alexandria University, Alexandria, Egypt; 52Research Center for Environmental Determinants of Health, Kermanshah University of Medical Sciences, Kermanshah, Iran; 53Associated Laboratory for Green Chemistry (LAQV), University of Porto, Porto, Portugal; 54Institute of Gerontology, National Academy of Medical Sciences of Ukraine, Kyiv, Ukraine; 55Department of Medical Parasitology, Abadan University of Medical Sciences, Abadan, Iran; 56Faculty of Medicine, Abadan University of Medical Sciences, Abadan, Iran; 57School of Public Health, Medical, and Veterinary Sciences, James Cook University, Douglas, QLD, Australia; 58Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC, Australia; 59Department of Epidemiology, Binzhou Medical University, Yantai City, China; 60Department of Pharmacology, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 61Obesity Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 62School of Health and Environmental Studies, Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates; 63School of Nursing and Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran; 64Independent Consultant, Tabriz, Iran; 65School of Business, London South Bank University, London, UK; 66Department of Biological Sciences, Ohio University, Zanesville, OH, USA; 67Department of Epidemiology and Biostatistics, Tehran University of Medical Sciences, Tehran, Iran; 68Pediatric Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran; 69Institute of Research and Development, Duy Tan University, Da Nang, Vietnam; 70Department of Computer Science, University of Human Development, Sulaymaniyah, Iraq; 71College of Science and Engineering, Hamad Bin Khalifa University, Doha, Qatar; 72Department of Community Medicine, University of Ibadan, Ibadan, Nigeria; 73Department of Community Medicine, University College Hospital, Ibadan, Ibadan, Nigeria; 74Faculty of Medicine, University of Belgrade, Belgrade, Serbia; 75Department of Epidemiology, University of Kragujevac, Kragujevac, Serbia; 76Department of Community Medicine, Dr. Baba Saheb Ambedkar Medical College & Hospital, Delhi, India; 77Department of Community Medicine, Banaras Hindu University, Varanasi, India; 78Vanke School of Public Health, Tsinghua University, Beijing, China; 79Institute of Molecular and Clinical Ophthalmology Basel, Switzerland, Basel, Switzerland; 80Department of Ophthalmology, Heidelberg University, Mannheim, Germany; 81Department of Family Medicine and Public Health, University of Opole, Opole, Poland; 82School of Management and Medical Informatics, Tabriz University of Medical Sciences, Tabriz, Iran; 83Department of Biostatistics, Abadan University of Medical Sciences, Abadan, Iran; 84Social Determinants of Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 85International Research Center of Excellence, Institute of Human Virology Nigeria, Abuja, Nigeria; 86Julius Centre for Health Sciences and Primary Care, Utrecht University, Utrecht, Netherlands; 87Department of Epidemiology and Biostatistics, Health Services Academy, Islamabad, Pakistan; 88Department of Population Science, Jatiya Kabi Kazi Nazrul Islam University, Mymensingh, Bangladesh; 89Faculty of Health and Wellbeing, Sheffield Hallam University, Sheffield, UK; 90College of Arts and Sciences, Ohio University, Zanesville, OH, USA; 91Department of Medical Parasitology, Cairo University, Cairo, Egypt; 92Department of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran; 93School of Traditional Chinese Medicine, Xiamen University Malaysia, Sepang, Malaysia; 94Independent Consultant, Jakarta, Indonesia; 95Imperial College Business School, Imperial College London, London, UK; 96Faculty of Public Health, University of Indonesia, Depok, Indonesia; 97Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy; 98HelpMeSee, New York, NY, USA; 99Mexican Institute of Ophthalmology, Queretaro, Mexico; 100Department of Health Sciences, University of Leicester, Leicester, UK; 101School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia; 102Clinical Research Development Center, Kermanshah University of Medical Sciences, Kermanshah, Iran; 103Campus Caucaia, Federal Institute of Education, Science and Technology of Ceará, Caucaia, Brazil; 104Department of Ophthalmology, Singleton Hospital, Swansea, UK; 105Peru Country Office, United Nations Population Fund (UNFPA), Lima, Peru; 106Clinical Microbiology and Parasitology Unit, Dr. Zora Profozic Polyclinic, Zagreb, Croatia; 107University Centre Varazdin, University North, Varazdin, Croatia; 108Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 109Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 110Department of Epidemiology and Biostatistics, Shahrekord University of Medical Sciences, Shahrekord, Iran; 111Department of Anatomical Sciences, Zanjan University of Medical Sciences, Zanjan, Iran; 112Health Systems and Policy Research Unit, Ahmadu Bello University, Zaria, Nigeria; 113Department of Health Care Management, Technical University of Berlin, Berlin, Germany; 114Department of Epidemiology, Arak University of Medical Sciences, Arak, Iran; 115Computer, Electrical, and Mathematical Sciences and Engineering Division, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia; 116Research and Analytics Department, Initiative for Financing Health and Human Development, Chennai, India; 117Department of Research and Analytics, Bioinsilico Technologies, Chennai, India; 118Department of General Surgery, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; 119Department of General Surgery, Emergency Hospital of Bucharest, Bucharest, Romania; 120Institute for Global Health Innovations, Duy Tan University, Hanoi, Vietnam; 121Administrative and Economic Sciences Department, University of Bucharest, Bucharest, Romania; 122Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, ON, Canada; 123Department of Psychiatry, University of Lagos, Lagos, Nigeria; 124Diplomacy and Public Relations Department, University of Human Development, Sulaymaniyah, Iraq; 125Department of Pharmacology and Therapeutics, University of Nigeria Nsukka, Enugu, Nigeria; 126Department of Health Metrics, Center for Health Outcomes & Evaluation, Bucharest, Romania; 127Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran; 128WHO Collaborating Centre for Public Health Education and Training, Imperial College London, London, UK; 129University College London Hospitals, London, UK; 130Department of Primary Care and Public Health, Imperial College London, London, UK; 131Academic Public Health England, Public Health England, London, UK; 132Department of Computer Science, Boston University, Boston, MA, USA; 133Epidemiology Research Unit (EPIUnit), University of Porto, Porto, Portugal; 134Department of Entomology, Ain Shams University, Cairo, Egypt; 135Independent Consultant, Karachi, Pakistan; 136Centre for Medical Informatics, University of Edinburgh, Edinburgh, UK; 137Division of General Internal Medicine, Harvard University, Boston, MA, USA; 138School of Medicine, University of Alabama at Birmingham, Birmingham, AL, UK; 139Medicine Service, US Department of Veterans Affairs (VA), Birmingham, AL, USA; 140Department of Ophthalmology, Hywel Dda University Health Board, Llanelli, UK; 141Department of Public Health, Erasmus University Medical Center, Rotterdam, Netherlands; 142Department of Community Medicine, Ahmadu Bello University, Zaria, Nigeria; 143Department of Global Health Research, Adaptive Knowledge Management, Victoria, BC, Canada; 144Department of Health Economics, Hanoi Medical University, Hanoi, Vietnam; 145Department of Molecular Medicine and Pathology, University of Auckland, Auckland, New Zealand; 146Clinical Hematology and Toxicology, Maurice Wilkins Centre, Auckland, New Zealand; 147Kasturba Medical College, Manipal Academy of Higher Education, Mangalore, India; 148Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy; 149Occupational Health Unit, Sant'Orsola Malpighi Hospital, Bologna, Italy; 150Center of Excellence in Behavioral Medicine, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam; 151Department of Diabetes and Metabolic Diseases, University of Tokyo, Tokyo, Japan; 152School of International Development and Global Studies, University of Ottawa, Ottawa, ON, Canada; 153The George Institute for Global Health, University of Oxford, Oxford, UK; 154Centre for Suicide Research and Prevention, University of Hong Kong, Hong Kong, China; 155Department of Social Work and Social Administration, University of Hong Kong, Hong Kong, China; 156Department of Neuropsychopharmacology, National Center of Neurology and Psychiatry, Kodaira, Japan; 157Department of Public Health, Juntendo University, Tokyo, Japan; 158Department of Epidemiology and Biostatistics, Wuhan University, Wuhan, China; 159School of Public Health and Management, Hubei University of Medicine, Shiyan, China; 160School of Public Health, Wuhan University of Science and Technology, Wuhan, China; 161Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology, Wuhan, China; 162School of Medicine, Wuhan University, Wuhan, China; 163Department of Health Education and Health Promotion, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Funding
This work was primarily supported by a grant from the Bill & Melinda Gates Foundation OPP1132415. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to study data and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Consortia
Contributions
EAC, MS, CAS, DMP, and SIH managed the estimation or publications process. EAC, CAS, EH, and KD wrote the first draft of the manuscript. CAS and EH had primary responsibility for applying analytical methods to produce estimates. KD, EH, CAS, KBJ, DMP, and EAC had primary responsibility for seeking, cataloging, extracting, or cleaning data; and designing or coding figures and tables. KBJ, JA1, VA1, OOA, MBA, FMA, TMA, DA, SCYA, MA1, JA2, MA2, MB, TWB, ZAB1, NLB, VKC, SMAD, GD, KD, SDD, AD, MESZ, AH, HH, CH, MH2, MH3, SSNI, JBJ, JJJ, LRK, NK1, GAK, NK2, KK, MMK, NK3, YJK, DK, VCL, MAM, FRM, CM, WM, MM, AM, SM2, AJN, IN, CTN, HLTN, BO, ATO, AOB, OEO, AP, VR, KR, RR, AMS, MAS, KS, JAS, WAS, BXT, BU, GTV, SY, NY, CY, AZ, and SIH provided data or critical feedback on data sources. CAS, MBA, DA, LE, MH2, MH3, NK3, MM, SM2, RR, AMS, AZ, and SIH developed methods or computational machinery. VA1, OOA, MBA, FMA, TMA, VA2, CLA, TA, DA, SCYA, JA2, MAJ, MA2, AAB, MB, TWB, KB, ZAB1, AB, OJB, NLB, ZAB2, VKC, SMAD, GD, FMD, KD, SDD, DD, MESZ, NAF, MF, RCF, YG, SH, HH, CH, TBH, MH2, MH3, OSI, IMI, MDI, SSNI, RPJ, JSJ, JBJ, JJJ, LRK, NK1, BKM, SEK, GAK, AKK, EAK, NK2, KK, MMK, NK3, YJK, SK, DK, SL, SM1, MAM, FRM, CM, WM, TM, MM, AM, SMM, SM2, MN, AJN, IN, CTN, HLTN, BO, ATO, AOB, OEO, AP, VR, DLR, SR, RR, AR, AIR, AMS, MAS, ES, SS, WAS, MBS, AJT, BXT, KBT, BU, FSV, GTV, TY, SY, PY, NY, CY, YY, MZ, YZ, ZZ, AZ, and SIH provided critical feedback on methods or results. CAS, MBA, CLA, TA, AAB, OJB, FC, GD, DD, MESZ, MET, NF, EF, NAF, MF, AH, MH1, MH3, OSI, IMI, MDI, EAK, NK3, CLV, VCL, PHL, TM, MM, SM2, RM, PM, KR, DLR, SR, AIR, AMS, AS, JAS, ES, MBS, FSV, MZ, AZ, and SIH drafted the work or revised it critically for important intellectual content. SIH managed the overall research enterprise. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study used de-identified data, and the waiver of informed consent was reviewed and approved by the University of Washington Institutional Review Board (application number 46665).
Consent for publication
Not applicable.
Competing interests
M Ausloos reports grants or contracts from the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI (research grant (oct 2018-sep 2022); role: team leader (Bucharest University of Economic Studies); grant title “Understanding and modelling time-space patterns of psychology-related inequalities and polarization”), outside the submitted work. T Bärnighausen reports research grants from the European Union (Horizon 2020 and EIT Health), German Research Foundation (DFG), US National Institutes of Health, German Ministry of Education and Research, Alexander von Humboldt Foundation, Else-Kröner-Fresenius-Foundation, Wellcome Trust, Bill & Melinda Gates Foundation, KfW, UNAIDS, and WHO; consulting fees from KfW for the OSCAR initiative in Vietnam; participation on a Data Safety Monitoring Board or Advisory Board with NIH-funded study “Healthy Options” (PIs: Smith Fawzi, Kaaya), Chair, Data Safety and Monitoring Board (DSMB), German National Committee on the “Future of Public Health Research and Education”, Chair of the scientific advisory board to the EDCTP Evaluation, Member of the UNAIDS Evaluation Expert Advisory Committee, National Institutes of Health Study Section Member on Population and Public Health Approaches to HIV/AIDS (PPAH), US National Academies of Sciences, Engineering, and Medicine’s Committee for the “Evaluation of Human Resources for Health in the Republic of Rwanda under the President’s Emergency Plan for AIDS Relief (PEPFAR)”, University of Pennsylvania (UPenn) Population Aging Research Center (PARC) External Advisory Board Member; leadership role of the Global Health Hub Germany (initiated by the German Ministry of Health) as a co-chair; all outside the submitted work. R C Franklin reports a leadership or fiduciary role in board, society, committee or advocacy group, paid or unpaid, with the Australasian College of Tropical Medicine as the Honorary Treasurer and Vice President; outside the submitted work. C Herteliu and A Pana report grants or contracts from Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project number PN-III-P4-ID-PCCF-2016-0084 (oct 2018-sep 2022) “Understanding and modelling time-space patterns of psychology-related inequalities and polarization” and project number PN-III-P2-2.1-SOL-2020-2-0351 (jun 2020-oct 2020) “Approaches within public health management in the context of COVID-19 pandemic”, all outside the submitted work. J S Ji reports consulting fees paid directly from The World Bank Group; honoraria from the Chinese Preventive Medicine Association and China Center for Disease Control and Prevention National Institute of Environmental Health Science, outside the submitted work. J Jozwiak reports payments from Teva Pharmaceuticals, Amgen, Synexus, Boehringer Ingelheim, ALAB laboratoria, and Zentiva as personal fees outside the submitted work. A Sheikh reports a research grant to their institutions from Health Data Research UK outside the submitted work. J A Singh reports consulting fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health and the American College of Rheumatology; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Simply Speaking; support for attending meetings and/or travel from OMERACT, an international organization that develops measures for clinical trials and receives arm’s length funding from 12 pharmaceutical companies, when traveling to OMERACT meetings; participation on a Data Safety Monitoring Board or Advisory Board as a member of the FDA Arthritis Advisory Committee; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, with OMERACT as a member of the steering committee, with the Veterans Affairs Rheumatology Field Advisory Committee as a member, and with the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis as a director and editor; stock or stock options in TPT Global Tech, Vaxart pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics, Inc., Seres Therapeutics and Charlotte’s Web Holdings Inc. and previously owned stock options in Amarin, Viking, and Moderna pharmaceuticals; all outside the submitted work. W A Stolk reports grants or contracts from the Bill and Melinda Gates Foundation (BMGF) as an active member of the NTD Modelling Consortium (grant number OPP1184344); consulting fees from Mectizan Donation Programme as a member of the Mectizan Expert Committee (MEC) in the form of a small honorarium for participating in MEC meetings; all outside the submitted work. All other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Appendix.
This file contains a GATHER compliance checklist, a list of data sources used in the present analysis, and further details of the analytical methodology and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schmidt, C.A., Cromwell, E.A., Hill, E. et al. The prevalence of onchocerciasis in Africa and Yemen, 2000–2018: a geospatial analysis. BMC Med 20, 293 (2022). https://doi.org/10.1186/s12916-022-02486-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02486-y