Abstract
Background and approach
Common standards for laboratory studies of non-target organisms are recognised as prerequisite to assist the risk assessments regarding the evaluation of environmental effects of transgenic crops. Here, we provide specific recommendations significant for experimental procedures of laboratory studies to test potential adverse effects of Bt maize on larvae of non-target Lepidoptera. We searched and analysed both ecotoxicological test protocols for pesticides in the EU as well as the non-target tests with Lepidoptera applied in unpublished industry studies submitted officially by agro-companies for the GMO authorisation in Europe.
Results
The classical ecotoxicology protocols applied for testing pesticides could serve as general guidelines, but do not completely fit the specific and differing requirements for assessing non-target effects of transgenic crops. The analysis of the non-target studies submitted for the application of the cultivation of Bt maize in Europe revealed critical limitations, thus corroborating the urgent need for common quality criteria. Based on our evaluations, we identified several issues requiring harmonisation or standardisation of the experimental conditions and approach, e.g., the application of Bt maize pollen, synthetic toxins, the provided diet for larvae, experimental controls, magnitude and duration of exposure to Bt, relevant variables to be recorded, and sufficient statistical power.
Conclusions
Our recommendations should stimulate the development of precise guidance for the authorities, and support the operationalisation of the required laboratory tests for the evaluation of non-target effects of Bt maize pollen on non-target Lepidoptera, also contributing to standards of other ecotoxicity tests with Lepidoptera larvae, e.g., for pesticides.
Similar content being viewed by others
Background
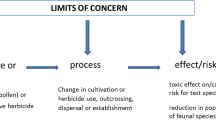
Genetically modified (GM) maize is one of the main transgenic crops cultivated worldwide [1]. A major application are Bt maize events modified with genes of the bacterium Bacillus thuringiensis. Bt maize produce insecticidal proteins [2], acting against insect herbivores of different taxa such as Coleoptera or Lepidoptera. Susceptible insects feeding on Bt maize, ingest the toxins and die. A further genetic modification of maize, often combined with insecticidal traits, confers herbicide resistance (HR) to certain broad-spectrum herbicide products such as glyphosate. Both HR and Bt maizes can potentially affect non-target Lepidoptera adversely. Wind dispersed and toxic Bt maize pollen can dust host plants of lepidopteran larvae, and larvae are affected lethally and sublethally when consuming this pollen inadvertently by feeding on the host plant tissue (e.g., [3,4,5,6]). The application of broad-spectrum herbicides in combination with HR crops is likely to change the herbicide regime, which can reduce the weed community within fields and in-field margins, in turn affecting larval and adult butterflies associated with such food plants (e.g., [7,8,9,10,11]).
With regard to Bt maize, the potential hazard imposed on non-target lepidopteran larvae was confirmed by an extensive review paper [12], concluding that knowledge on Bt maize pollen effects on lepidopteran larvae is rather deficient. Lang and Otto [12] recommended carrying out more laboratory studies examining effects of Bt maize pollen on non-target lepidopterans, in particular emphasising the need for more realistic, ecologically meaningful studies and the urgent demand for an international standardisation of such experiments. However, since 2010, only one further research paper about Bt maize pollen effects on non-target Lepidoptera has been published [6]. Other recent studies reporting on Bt effects on Lepidoptera larvae focused on Bt sprays instead of Cry proteins or Cry maize pollen [13], studied lepidopteran pest species and not non-target species [14,15,16,17,18], were done with transgenic crops other than maize [19], investigated nectar feeding rather than pollen feeding [20], or studied exposure rather than direct toxic effects [21, 22].
Including the one new study, possible adverse effects of Bt maize pollen feeding on non-target lepidopteran larvae have been studied for twelve species worldwide (including four secondary pests such as Pieris spp.). This sample is remarkably low compared to the taxonomic diversity of the 160,000 described or half a million estimated Lepidoptera species worldwide [23]. In consequence, the knowledge gap, critical for the assessment of effects from Bt maize cultivation on Lepidoptera biodiversity, reported by Lang and Otto [12] still exists.
Potential risks that the cultivation of GM crops pose to non-target organisms (NTO) are subject to pre-release risk assessments, and are a mandatory part of the authorisation process in Europe (e.g., [24,25,26]) and many other countries. Despite this, a common standard, or guidelines for laboratory studies have not been established for tests with Lepidoptera larvae (or other NTO), notwithstanding the recognised need for such standardisation in the GM crop risk assessment [25,26,27,28]. In Europe, the European Food Safety Authority (EFSA) as the competent authority for the GMO risk assessment provides general guidelines for the GMO risk assessment of NTOs. The EFSA guidelines [25,26,27] specify a stepwise, tiered approach to test non-target effects. Laboratory studies of non-target organisms inform the hazard characterisation, and are termed first or early-tier studies. Although some and general requirements for such first-tier tests are expressed by EFSA [25], applicants can develop their own protocols and are not obliged to follow standardised protocols as it is e.g., the norm in the risk assessment of pesticides [29,30,31,32,33]. Indeed such protocols do not exist, and, accordingly, test protocols and study designs submitted for GMO applications vary greatly leading to a considerable diversity in scope and quality of first-tier tests. In consequence, uncertainties arise for both applicants and authorities with regard to required ecotoxicological parameters and the way how to measure them. These uncertainties impact the environmental risk assessment and risk management measures substantially [34, 35]. Several recommendations have been published dealing with required standards for GMO first-tier tests in the laboratory with non-target organisms [28, 36, 37], but none addressed test design and quality criteria for assessing a Bt maize effect on lepidopteran larvae. As insect resistance against Lepidoptera is frequently applied in Bt crops, and stacking different toxins in a single plant event is increasing [38], larvae of Lepidoptera belong to the non-target organisms which face the highest risk from exposure to Bt maize cultivation.
To improve current test protocols, we checked the study designs for non-target tests with Lepidoptera submitted for GMO authorization under Regulation (EC) No 1829/2003 of the European Parliament and of the council of 22 September 2003 on genetically modified food and feed Regulation 1829/2013, also taking into account current ecotoxicological test protocols for pesticides in the EU [39]. Based on these analyses, we provide guidance and recommendations on the experimental design and required standards for early-tier laboratory studies studying the effect of lepidopteran-toxic Bt maize on larvae of Lepidoptera.
Methods
Laboratory studies with lepidopteran larvae
So far, a range of transgenic Bt maize events targeting Lepidoptera pests have been submitted for cultivation in Europe. As many transgenic Bt maize events are potentially harmful to the larvae of butterflies and moths (see above), these applications require the specific assessment of effects on non-target Lepidoptera. Companies applying for the cultivation of GM crops in Europe, therefore, provide information and results of ecotoxicological studies with non-target Lepidoptera and Bt to the EFSA [25, 26]. While some of the information submitted is from public, peer-reviewed studies, most of the reports include unpublished studies specifically prepared by companies to assist an application.
The data base of the Federal Agency for Nature Conservation (BfN), Bonn, Germany, was searched to recover the company studies relevant for the analysis. The BfN is a scientific authority and legally appointed to assist the environmental risk assessment of GM crops, has access to data delivered to EFSA in GMO applications, and maintains a meta-database which allows to search for industry studies. We searched the BfN database to identify the studies submitted by applicants to the European Union, using multiple appropriate search strings and keywords such as {*larva* or *lepi* or *effica* or *butter* or *lepidopter*}. The search results were subsequently checked for relevant studies. Then, the methodology applied in the studies, but not the results, were analysed further.
NTO testing for chemical plant protection products
We analysed the NTO testing protocols which are required by the current EU legislation as well as further published guidelines to assess the ecotoxicity of chemical plant protection products (PPP). The ecotoxicological risk assessment for PPP is legally based on the Commission Regulation (EU) No 283/2013 of 1 March 2013, setting out the data requirements for active substances, and (EU) No 284/2013 of 1 March 2013, setting out the data requirements for PPP, as well as Commission Regulation (EU) No 546/2011 of 10 June 2011 regarding uniform principles for evaluation and authorisation of PPP in accordance with Regulation (EC) No 1107/2009 of 21 October of the European Parliament and of the Council. Further relevant guidelines include the scientific opinions of the European Food Safety Authority (EFSA) [27, 33], guidelines published by the Society of Environmental Toxicology and Chemistry (SETAC) [39], by the European Commission [40], and by the European and Mediterranean Plant Protection Organization (EPPO) [41].
We compared the standardisation requirements for NTO testing of chemical PPPs with the above industry protocols of the tests with lepidopteran larvae which were used for the risk assessment of Bt maize. Through this comparison, we identified relevant aspects and parameters, which are already established in chemical PPP assessment but are lacking in the GMO test protocols.
Recommendations for standardising laboratory tests for Bt maize effects on NT lepidopteran larvae
Synthesising the above analyses of the industry studies and the chemical PPP legislation and regulation, we conclude on and list general recommendations for harmonising and improving the procedures for testing the effects of insecticidal Bt maize pollen on larvae of Lepidoptera. Our recommendations are specific for tests with Lepidoptera but include several other sources to improve non-target testing of GMO (e.g., [25, 26, 28, 36, 37, 42]) as well as our own experiences with NTO testing (e.g., [4, 12, 43]). By doing so, we provide specific advice for the experimental design and procedure of early-tier testing of effects on non-target Lepidoptera, rather than addressing a more general framework of GMO risk assessment involving conceptual aspects such as hazard identification, exposure assessment, or risk characterisation.
Results
Laboratory studies with lepidopteran larvae used in GMO applications
In total, we retrieved 14 industry studies reporting on the effect of Bt on Lepidoptera larvae (see Additional file 1: Table S1). The studies were submitted by a range of companies but were all carried out in the USA. Of those studies, nine studies were conducted with target species, and five studies with non-target species. In the NTO studies, only two different species were used: the Monarch butterfly (Danaus plexippus) and the Painted Lady (Vanessa cardui). In contrast, seven target species were tested (Agrotis ipsilon, Diatraea grandiosella, Diatraea saccharalis, Elasmopalpus lignosellus, Helicoverpa zea, Ostrinia nubilalis, Spodoptera frugiperda). Eleven studies involved treatments differing in Bt doses, thus enabling the calculation of a dose–response relationship, while the three remaining studies analysed the equivalence or efficacy of a tested Bt event and did not consider different Bt toxin concentrations.
Various Cry proteins were tested, mainly mixed into an artificial diet for NTOs; only in one NTO study plant material was used as larval diet (Table 1). The natural exposure of NTO larvae to Bt maize, i.e. to maize pollen, was studied in 19% of all experiments conducted in the studies. The major variables tested were survival and larval body weight. Notably, no parameter for reproductive output was ever measured. It was remarkable that the duration of the experiments was rarely longer than the exposure period to the Bt toxin, thus any delayed effects were not detectable (cf. [4]).
In general, effects on target organisms (TO) are regularly tested to evaluate the efficacy of a Bt maize event for pest control. Such results of TO may provide helpful information as it might supply indirect indications of some Bt maize effects on NTO. Nevertheless, a direct testing of NTO lepidopteran larvae is crucial; however, only five studies qualified as genuine NTO studies testing the two NTO species Danaus plexippus and Vanessa cardui. In these five NTO studies, several critical aspects were identified affecting the significance of the results:
-
(i)
in only 3 of 5 studies were the larvae exposed to Bt maize pollen, the acting agent in the field, the other studies used isolated Cry toxins;
-
(ii)
only one study used the natural exposure route, i.e. Bt maize pollen applied to the surface of host plant leaves, but failed to record the Bt concentration in the pollen used for the experiments;
-
(iii)
two studies did not assess explicitly the degree of the naturally occurring pollen exposure prior to the start of their experiments, which would be necessary to justify the applied doses;
-
(iv)
the effective Bt intake by the larvae was never observed or recorded directly, thus only concluded indirectly in cases where adverse effects were observed; hence, in the only study reporting no adverse effects of Bt consumption, successful uptake of Bt by larvae remains unclear;
-
(v)
no quantification was done of the exact amount of Bt actually consumed and ingested by larvae, thus a dose–response (e.g., LD50) could not be calculated;
-
(vi)
only one study ran positive controls, testing a control species sensitive to Bt, thus allowing to assess the bioactivity of the applied Bt toxin, and testing a known toxic substance other than Bt, thus confirming the suitability of the experimental approach;
-
(vii)
except for one study, all remaining studies stopped the experiments with the termination of exposure, hence any chronic and delayed effects known to occur due to prior Bt exposure could not be detected;
-
(viii)
none of the studies recorded sublethal effects on adults or any parameter of reproductive output;
-
(ix)
in four of the five studies, only the absolute larval mass at the end of the study was recorded, but not initial body mass allowing to calculate weight gain (body mass of larvae is highly variable, thus not standardising weight increase between treatments can mask existing effects on body weight);
-
(x)
in 50% of the studies the origin and source of the used larvae were not mentioned;
-
(xi)
remarkably, in the 3 experiments studying Vanessa cardui, the larvae of this day-active species were kept in complete darkness during the study, with unknown consequences on their feeding behaviour and Bt uptake;
-
(xii)
in some cases, the presentation and statistics of the results were insufficient, e.g., only means but no variance presented, unclear sample sizes per treatment, or even no data shown for a given statement.
Overall, the major shortcomings of the analysed studies included too few NTO species, no ecological realism in exposure to and consumption of the Bt toxin, no control of effective Bt intake by the larvae, lack of appropriate endpoints such as sublethal effects and reproduction parameters, and to some extent limitations in the statistical analysis. Thus, the above limitations weaken considerably the significance of the studies for the risk assessment of the possible adverse effects of Bt maize pollen on lepidopteran larvae.
NTO testing for chemical plant protection products
Currently, standard test protocols for Lepidoptera are missing in the ecotoxicological risk assessment for chemical plant protection products (PPP). However, EFSA [33] considers Lepidoptera to be important drivers for the ecosystem services pollination and food web support, and recommends including an oral toxicity study with Lepidopteran larvae as representative of herbivorous species. EFSA [33] also recommends developing new test methods that would include effects from chronic exposure and delayed effects in non-target arthropods in the lower tiers. This should allow for estimating effects on the most crucial life history parameters, such as longevity and reproduction rate.
In general, for chemical PPP basic data of non-target arthropods are required from honey bees and other non-target organisms such as e.g., mites and parasitoid wasps, but not from lepidopteran species. Dependent on the first results, if risks are indicated, then further species should be tested, e.g., predatory bugs (Heteroptera), lacewings (Neuroptera) or ladybird beetles (Coleoptera) according to the so-called ESCORT 2 [39]. The effects considered in the risk assessment of PPP are mortality as well as sublethal effects including adverse effects on reproduction data. Basically, the following parameters are to be recorded: acute oral and contact toxicity, chronic toxicity (in adult honey bees), development and brood activity (honey bees), and sublethal effects such as behavioural and reproductive effects (Commission regulation (EU) No 283/2013). The selection of the test species should be related to the potential use of the plant protection products (e.g., foliar or soil application).
The risk for products applied as sprays has to be assessed for in-field and off-field scenarios, where in-field is based on the application rate and off-field on drift rates [27, 40, 41]. To account for multiple applications a Multiple Application Factor (MAF) must be applied. The current off-field risk assessment for non-target arthropods also includes a correction factor of 10 to cover inter-species variability since a limited number of indicator species are tested when compared to the range of species, which could be exposed in off-field habitats [33, 39].
In view of the current practice of chemical PPP testing, the above assessed industry studies, treating the effect of Bt maize pollen on larvae of Lepidoptera, fell short in various respects (see section above). Summing up, the current regulatory guidelines for testing the impact of PPP on NTO include the following components, providing valuable and important indications for GMO testing:
-
(i)
consideration of Lepidoptera as an important ecological group,
-
(ii)
development of harmonised test methods for laboratory studies with Lepidoptera,
-
(iii)
laboratory tests based on the expected exposure in off-field habitats,
-
(iv)
testing of sublethal effects including effects on reproduction,
-
(v)
testing acute (immediate) and chronic (delayed) effects,
-
(vi)
consideration of the way that a PPP is used, and of the resulting exposure of NTO,
-
(vii)
accounting for additive, multiple applications affecting NTO, and
-
(viii)
safety factors to allow for uncertainty caused by inter-species variability of NTO.
Recommendations for standardising laboratory tests for Bt maize effects on NT lepidopteran larvae
In general, we recommend that the laboratory studies and methods should be harmonised as far as possible to ensure the standardisation, reproducibility and quality of the studies [26]. Ring tests with several laboratories would also be beneficial, i.e. experiments and samples should be carried out by different laboratories at the same time, which serves as an external quality assurance (e.g., [44]). All experimental, chemical and physical conditions relevant for the study must be described and justified, such as air temperature, humidity, day–night cycle, and more, see below [26]. In the following we focus our recommendations on important aspects of practical testing to ensure sufficient quality of experiments, summarised in Table 2.
Bt maize pollen
In contrast to many chemical insecticides, Bt proteins are gut toxins and require ingestion by the organisms. Therefore, testing requires the ingestion of the Bt via food. The Bt test substance should be applied to the larvae in a way as natural as possible, i.e. similar to the occurring exposure under field conditions [28]. Preferably, the larvae should be fed freshly collected Bt maize pollen instead of (microbially produced) Cry proteins or Cry powders. Applying fresh maize pollen will often not be possible, thus frozen and stored maize pollen can be used thawed for experiments shortly before (< 1 day before the experiment). However, storage temperature of pollen is critical to prevent the degradation of the Bt toxin in the pollen, and Nguyen and Jehle [45] recommend a storage temperature of Bt proteins between − 20 °C and − 80 °C depending on the duration of storage and the acceptable loss of activity.
The applied Bt maize pollen must be well characterized and described. This includes quantifying the Bt concentration in the pollen and the stability of the Bt protein [36], to allow calculation of the exact exposure concentration or dose (see also [25, 26]). The same batch of pollen should be used throughout the experiment, otherwise new batches must be characterised fully [36]. The use of ELISA tests to quantify Bt concentration in pollen requires the implementation of standard procedures with regard to sample preparation, reference material (particularly the toxin used for the standards curve), laboratory equipment, analytical protocol, and analysis of results (for more details see [44], and references therein).
Artificial Bt toxins
Complementary to Bt maize pollen, artificial Bt toxins (e.g., cloned in E. coli) instead of Bt maize pollen may be used in some instances. Artificial Bt toxins may be useful (i) in a first screening of a range of species as this requires lower effort than the above testing with maize pollen, (ii) if a range finding of doses is necessary to identify the different doses required for the testing of a dose–response, (iii) when worst-case conditions must be tested, or (iv) where the pollen of the respective transgenic maize contains very small amounts of Cry protein [36]. In all cases where artificial Bt toxins are used the equivalence with the in-planta protein must be confirmed by both the analysis of length and sequence of the toxin and by bioassays. Moreover, it must be confirmed that the Bt mixed into an artificial diet is distributed homogeneously and results in the expected concentration [36]. We strongly recommend supplementing experiments using artificial diets with additional studies under near-natural conditions.
Matrix (diet)
Preferably, the leaves or plants of the respective host plants should be used as the basic (matrix) diet for the larvae instead of artificial diets. This serves to mimic the natural situation as much as possible, thus increasing the validity and relevance of the results. In addition, the respective calculations to convert µg toxin per volume of artificial diet into consumption of pollen per leaf surface area may be quite imprecise, and add another source of uncertainty to the assessment. Only in cases where larvae are expected to use leaf or root tissue as a food source, parts of the maize plant itself should be used in experiments.
Commonly, leaf discs cut out of the respective host plants are used as the basic matrix instead of whole plant leaves or whole host plants [4, 6]. When using leaf discs, a recommended approach is to put the pollen-dusted discs into the wells of a micro-plate together with one larva, the ground of the wells covered with agar to prevent desiccation of the leaf discs [5]. Wells must match the size of the respective larvae, otherwise too small wells may cause additional mortality or other adverse effects on larvae. Using whole host plants or whole leaves has been recommended [28], because cutting plants can elicit physiological plant defence reactions unfavourable for lepidopteran larvae. However, a whole leaf or whole plant approach has been applied only occasionally so far (e.g., [46, 47]). Regardless whether using leaf discs or whole leaves/plants, it is necessary to standardise plant quality as much as possible i.e. using the same plant variety in all trials, assuring a standardised breeding and cultivation of all host plants used, and equal age of plant material used in the actual experiments. This is important because plant differences can affect the constitution and development of the larvae [46].
Using artificial diets rather than plant material makes it easier to standardise experimental conditions, but diet composition may influence effects of Bt toxins on larvae. For instance, a high concentration of carotenoids can decrease larval mortality due to Bt [48]. Furthermore, it should be ensured that no antibiotics are added to the diet as this can influence the Bt effect on larvae, too [49, 50].
Test species
Detailed information must be given of origin and/or source of test organisms, which are preferably reared and bred in the laboratory [36]. In case of field collected specimens, information on the site and method of collection as well as details on the handling and maintenance between the time of collection and use in the experiments should be given (e.g., [29, 36]). The selection of test species should involve preferably those European species exposed to Bt maize pollen shedding, and account for possible regional differences and protected species [12, 25, 26, 36, 51]. The life stages most susceptible to Bt should be studied, which is generally the first instar. Older instars can be used if 1st instars are unfeasible to handle (e.g., due to small body size) or are not exposed (e.g., because of endophytic life style).
Controls
In addition to the quantification of the Bt (see above), the bioactivity of the Bt maize pollen must be verified (= positive control) by feeding the Bt pollen to a sensitive lepidopteran species with known susceptibility. The positive control serves to prove the exposure of the test organisms and the successful ingestion of Bt maize pollen, and demonstrates that the test system is appropriate to detect adverse effects [12, 36, 37]. Successful ingestion of Bt may also be verified by analysing the larvae with immunodetection techniques such as ELISA or Western Blotting, however, a positive test does not necessarily prove the bioactivity of the Bt maize pollen.
Negative controls include a treatment where no adverse effects are expected. In tests with artificial diets, the diet is supplied without Bt toxin mixed in it. In Bt pollen tests, a host plant only and a treatment with conventional maize pollen (dusted on host plant) may be used. If feasible, non-transgenic maize pollen should be used from isolines of the respective Bt maize event. However, such isolines are often not freely available on the market, and reference material may not be provided by the companies. In this case, pollen from other conventional maize should be used instead. Negative control treatments assess the background influences of the test system, i.e. by checking the suitability of the test system, the organisms (e.g., for health) and the test conditions, and detect potential effects of the matrix, i.e. the host plant, control pollen or diet [28, 36]. By convention, mortality of the negative control should not exceed 20%, proving that general test conditions and state of test organisms are appropriate and do not affect tests results [26].
Magnitude of exposure
The chosen maize pollen densities of the test trials must be justified explicitly to mirror the range of the natural experienced exposure pollen densities [4, 28, 36]. In addition, treatments exceeding the naturally expected exposure should be included, and the chosen treatments should allow for deriving a dose/exposure–response curve and LD/LC50 values [25, 26]. It is recommended to conduct a preliminary, simple range finding test to identify the required pollen densities for the calculation of the respective dose–response. It has to be noted that, for the exact determination of the dose uptake and of LD values, the amount of Bt pollen ingested by larvae has to be recorded, e.g. by an ELISA or by recording the number of pollen eaten [4, 12, 20].
Duration of exposure
In general, the duration of exposure should reflect the naturally occurring exposure period. In the case of pollen-shedding maize plants, this will result in a consistent exposure of several days depending on the pollen-shedding period of the respective maize event in a given receiving environment [12, 37, 52]. It is important to account for acute and chronic exposure, i.e. studying a short but high exposure to Bt with immediate effects as well as a lower Bt exposure spread over a longer period with delayed effects. As maize pollen can be shed for 2 weeks for one field, or up to 6 weeks in a given maize-growing region [53, 54], we recommend including an exposure treatment covering the entire larval period. For reasons of comparisons between different lepidopteran species, we suggest standard exposure times which should be applied to all tests with Bt proteins, e.g., 2 days, up to 9 days and entire larval period. In any case, the chosen exposure durations and pollen densities must be justified explicitly.
Duration of experiment
The studies must last longer than exposure time to record potential delayed Bt effects and to account for parameters of the adult stage [4, 12, 55]. We recommend to test the whole larval period (if feasible), and to record parameters or correlates of adult fitness such as egg numbers or body size (see endpoints below).
Endpoints
Variables measured should include mortality of larvae and sublethal effects such as body mass, development time, feeding activity, and in particular parameters of the adults, e.g., fecundity or adult body size [4, 12, 18, 25]. Differing doses and sample size should enable the exact calculation of LC/LD or EC/ED values [26]. Effects on generational relative fitness (fecundity, reproduction) are particularly important endpoints, because adverse effects of transgenic plants on populations of non-target species would occur through some component of fitness [12, 26, 55, 56].
Sample size
Replication must be sufficient to allow the detection of adverse Bt effects of a given effect size with a desired probability. A prospective power analysis is mandatory to ensure appropriate replication to detect a pre-defined effect size [12, 36, 57,58,59], retrospective power analyses are methodologically not possible [26, 60]. In general, an 80% power at an alpha level of α = 0.05 is recommended [34, 59]. EFSA [26] mentions that experiments should be able to detect an effect size of < 20%, this effect size acting as a trigger value for further, higher-tier studies.
Conclusions
Clearly, minimum standard criteria of toxicology tests with non-target organisms must be met for studies to qualify as sufficiently robust to be relevant for the environmental risk assessment of GMOs [37]. In Europe, a comprehensive body of legislation framework exists regulating the release of genetically modified organism (GMO) (e.g., [24, 40]). EFSA assesses each application for release of a GMO, and regularly publishes guidelines addressing relevant issues including the testing and evaluation of possible harmful GMO effects (e.g., [25]). Although some minimum criteria have been communicated for non-target organism studies to qualify as relevant information for the risk assessment [26], no common standardised pre-release testing protocols exist or have been agreed on for non-target laboratory studies with GMO. This is in clear contrast to the ecotoxicological risk assessment for plant protection products.
Tests with GMO have to meet requirements differing from tests for chemical plant protection products, due to their specific characteristics [51, 61, 62]. However, the presented case examples revealed critical methodological limitations of such tests, and demonstrated the unwanted consequences of missing quality criteria. Our analysis supports the request for mandatory definitions, standards and quality criteria for GMO pre-release testing of non-target organisms [28, 36]. Such standards are essential to assess the potential harm of Bt maize on NT Lepidoptera and to provide confidence in the pre-release environmental risk assessment (see also [28]). In this respect, classical ecotoxicological protocols for testing pesticides could serve as general guidelines, but do not completely fit the specific requirements for assessing GMO effects, e.g., a whole plant approach [62], or oral exposure of Bt maize pollen. By presenting the necessary quality criteria for testing the effect of Bt maize pollen consumption on larvae of butterflies and moths, this publication can serve as a common base when planning studies with lepidopteran larvae and Bt maize to ensure quality, reliability, robustness and replicability of the respective experiments. As such, our recommendations should stimulate more precise guidance to the authorities and support further operationalisation.
Availability of data and materials
Restrictions to the public availability apply to some of the sources that support the findings of this study, in particular the contents of the unpublished regulatory industry studies are subject to obligations of confidentiality. According to European and national law on the access to environmental information, the studies used in our analyses may be requested from the respective authorities, including the Federal Agency for Nature Conservation (BfN).
Abbreviations
- BfN:
-
Bundesamt für Naturschutz
- Bt:
-
Bacillus thuringiensis
- EC:
-
effective concentration
- ED:
-
effective dose
- EEC:
-
expected environmental concentration
- EFSA:
-
European Food Safety Authority
- EPPO:
-
European and Mediterranean Plant Protection Organization
- EU:
-
European Union
- GM:
-
genetically modified
- GMO:
-
genetically modified organism
- HR:
-
herbicide resistant
- LC:
-
lethal concentration
- LD:
-
lethal dose
- MAF:
-
multiple application factor
- NT:
-
non target
- NTO:
-
non-target organism
- PPP:
-
plant protection products
- SD:
-
standard deviation
- SETAC:
-
Society of Environmental Toxicology and Chemistry
- TO:
-
target organism
References
ISAAA International Service for the Acquisition of Agri-biotech Applications (2016) Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52. Ithaca, ISAAA
Glare TR, O’Callaghan M (2000) Bacillus thuringiensis: biology, ecology and safety. Wiley, Chichester
Zangerl AR, McKenna D, Wraight CL, Carroll M, Ficarello P, Warner R, Berenbaum MR (2001) Effects of exposure to event 176 Bacillus thuringiensis corn pollen on monarch and black swallowtail caterpillars under field conditions. Proc Natl Acad Sci USA 98:11908–11912
Lang A, Vojtech E (2006) The effects of pollen consumption of transgenic Bt maize on the common swallowtail, Papilio machaon L. (Lepidoptera, Papilionidae). Basic Appl Ecol 7:296–306
Felke M, Langenbruch GA, Feiertag S, Kassa A (2010) Effect of Bt-176 maize pollen on first instar larvae of the peacock butterfly (Inachis io) (Lepidoptera; Nymphalidae). Environ Biosaf Res 9:5–12
Schuppener M, Mühlhauser J, Müller A-K, Rauschen S (2012) Environmental risk assessment for the small tortoiseshell Aglais urticae and a stacked Bt-maize with combined resistances against Lepidoptera and Chrysomelidae in central European agrarian landscapes. Mol Ecol 21:4646–4662
Haughton AJ, Champion GT, Hawes C, Heard MS, Brooks DR, Bohan DA, Clark SJ, Dewar AM, Firbank LG, Osborne JL, Perry JN, Rothery P, Roy DB, Scott RJ, Woiwod IP, Birchall C, Skellern MP, Walker JH, Baker P, Browne EL, Dewar AJG, Garner BH, Haylock LA, Horne SL, Mason NS, Sands RJN, Walker MJ (2003) Invertebrate responses to the management of genetically modified herbicide tolerant and conventional spring crops. II. Within-field epigeal and aerial arthropods. Philos Trans R Soc Lond Biol Sci 358:1847–1862
Pleasants JM, Oberhauser KS (2012) Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv Divers 6:135–144
Roy DB, Bohan DA, Haughton AJ, Hill MO, Osborne JL, Clark SJ, Perry JN, Rothery P, Scott RJ, Brooks DR, Champion GT, Hawes C, Heard MS, Firbank LG (2003) Invertebrates and vegetation of field margins adjacent to crops subject to contrasting herbicide regimes in the farm scale evaluations of genetically modified herbicide-tolerant crops. Philos Trans R Soc Lond B Biol Sci 358:1879–1898
Stenoien C, Nail KR, Zalucki JM, Parry H, Oberhauser KS, Zalucki MP (2018) Monarchs in decline: a collateral landscape-level effect of modern agriculture. Insect Sci 25:528–541
Thogmartin WE, Wiederholt R, Oberhauser K, Drum RG, Diffendorfer JE, Altizer S, Taylor OR, Pleasants J, Semmens D, Semmens B, Erickson R, Libby K, Lopez-Hoffman L (2017) Monarch butterfly population decline in North America: identifying the threatening processes. R Soc Open Sci 4:170760
Lang A, Otto M (2010) A synthesis of laboratory and field studies on the effects of transgenic Bt-maize on non-target Lepidoptera. Entomol Exp Appl 135:121–134
Burgess EPJ, Barraclough EI, Kean AM, Markwick NP, Malone LA (2015) Responses of 9 lepidopteran species to Bacillus thuringiensis: how useful is phylogenetic relatedness for selecting surrogate species for nontarget arthropod risk assessment? Insect Sci 22:803–812
Erasmus A, Van Rensburg JBJ, Van den Berg J (2010) Effects of Bt maize on Agrotis segetum (Lepidoptera: Noctuidae): a pest of maize seedlings. Environ Entomol 39:702–706
Mahmoud AMA, De Luna-Santillana EJ, Rodríguez-Perez MA (2011) Parasitism by the endoparasitoid, Cotesia flavipes induces cellular immunosuppression and enhances susceptibility of the sugar cane borer, Diatraea saccharalis to Bacillus thuringiensis. J Insect Sci 11:119
Pérez-Hedo M, López C, Albajes R, Eizaguirre M (2012) Low susceptibility of non-target Lepidopteran maize pests to the Bt protein Cry1Ab. Bull Entomol Res 102:737–743
Erasmus A, Van den Berg J (2014) Effect of Bt-maize expressing Cry1Ab toxin on non-target Coleoptera and Lepidoptera pests of maize in South Africa. Afr Entomol 22:167–179
Muñoz P, López C, Moralejo M, Pérez-Hedo M, Eizaguirre M (2014) Response of last instar Helicoverpa armigera larvae to Bt toxin ingestion: changes in the development and in the CYP6AE14, CYP6B2 and CYP9A12 gene expression. PLoS ONE 9(6):e99229. https://doi.org/10.1371/journal.pone.0099229
Kjær C, Damgaard C, Lauritzen AJ (2010) Assessment of effects of Bt-oilseed rape on large white butterfly (Pieris brassicae) in natural habitats. Entomol Exp Appl 134:304–311
Paula DP, Andow DA, Timbo RV, Sujii ER, Pires CSS et al (2014) Uptake and transfer of a Bt toxin by a Lepidoptera to its eggs and effects on its offspring. PLoS ONE 9(4):e95422. https://doi.org/10.1371/journal.pone.0095422
Lang A, Otto M (2015) Feeding behaviour on host plants may influence potential exposure to Bt maize pollen of Aglais urticae larvae (Lepidoptera, Nymphalidae). Insects 6:760–771
Lang A, Oehen B, Ross JH, Bieri K, Steinbrich A (2015) Potential exposure of butterflies in protected habitats by Bt maize cultivation: a case study in Switzerland. Biol Conserv 192:369–377
Kristensen NP, Scoble MJ, Karsholt O (2007) Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity. Zootaxa 1668:699–747
EC European Community (2001) Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EC—Commission Declaration. Off J Eur Communities L106:1–39
EFSA European Food Safety Authority (2010) Guidance on the environmental risk assessment of genetically modified plants. Scientific opinion of the EFSA panel on genetically modified organisms (GMO). EFSA J 8:1879
EFSA European Food Safety Authority (2010) Scientific opinion on the assessment of potential impacts of genetically modified plants on non-target organisms. EFSA J 8(11):1877
EFSA European Food Safety Authority (2013) EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J 11(7):3295
Hilbeck A, Jänsch S, Meier M, Römbke J (2008) Analysis and validation of present ecotoxicological test methods and strategies for the risk assessment of genetically modified plants. BfN-Skripten 236:1–143
OECD Organisation for Economic Co-operation and Development (1998) OECD series on principles of good laboratory practice and compliance monitoring Number 1. OECD Principles on Good Laboratory Practice (as revised in 1997). Organisation for Economic Co-operation and Development, Paris
OECD Organisation for Economic Co-operation and Development (1999) OECD series on principles of GLP and compliance monitoring number 4 (revised). Organisation for Economic Co-operation and Development, Paris
OECD Organisation for Economic Co-operation and Development (1999) OECD series on principles of GLP and compliance monitoring number 7 (revised). Organisation for Economic Co-operation and Development, Paris
OECD Organisation for Economic Co-operation and Development (2005) Good laboratory practice. OECD principles and guidance for compliance monitoring, Organisation for Economic Co-operation and Development
EFSA European Food Safety Authority (2015) Scientific opinion addressing the state of the science on risk assessment of plant protection products for non-target arthropods. EFSA J 13(2):3996
Perry JN, Devos Y, Arpaia S, Bartsch D, Gathmann A et al (2010) A mathematical model of exposure of nontarget Lepidoptera to Bt-maize pollen expressing Cry1Ab within Europe. Proc R Soc B277(1686):1417–1425
Fahse L, Papastefanoua P, Otto M (2018) Estimating acute mortality of Lepidoptera caused by the cultivation of insect-resistant Bt maize—the LepiX model. Ecol Model 371:50–59
Romeis J, Hellmich RL, Candolfi MP, Carstens K, De Schrijver A, Gatehouse AMR, Herman RA, Huesing JE, McLean MA, Raybould A, Shelton AM, Waggoner A (2011) Recommendations for the design of laboratory studies on non-target arthropods for risk assessment of genetically engineered plants. Transgenic Res 20:1–22
De Schrijver A, Devos Y, De Clercq P, Gathmann A, Romeis J (2016) Quality of laboratory studies assessing effects of Bt-proteins on non-target organisms: minimal criteria for acceptability. Transgenic Res 25:395–411
Parisi C, Tillie P, Rodríguez-Cerezo E (2016) The global pipeline of GM crops out to 2020. Nat Biotechnol 34(1):31–36
Candolfi MP, Barrett KL, Campbell PJ, Forster R, Grandy N, Huet M-C, Lewis G, Oomen PA, Schmuck R, Vogt H (2001) Guidance document on regulatory testing and risk assessment procedures for plant protection products with non-target arthropods: from the Escort 2 workshop (European standard characteristics of non-target arthropod regulatory testing). SETAC Press, Pensacola, pp 1–46
EC European Commission (2002): Guidance document on terrestrial ecotoxicology under council directive 91/414/EEC SANCO/10329/2002 rev 2 final, 17 October 2002, p 39
EPPO European and Mediterranean Plant Protection Organization (2010) Side-effects on honeybees. Bulletin OEPP/EPPO Bulletin 40:313–319
Römbke J, Jänsch S, Meier M, Hilbeck A, Teichmann H, Tappeser B (2010) General recommendations for soil ecotoxicological tests suitable for the environmental risk assessment of genetically modified plants. Integr Environ Assess Manag 6:287–300
Ludy C, Lang A (2006) Bt maize pollen exposure and impact on the garden spider, Araneus diadematus. Ent Exp Appl 118:145–156
Székács A, Weiss G, Quist D, Takács E, Darvas B, Meier M, Swain T, Hilbeck A (2012) Inter-laboratory comparison of Cry1Ab toxin quantification in MON 810 maize by enzyme-immunoassay. Food Agric Immun 23:99–121
Nguyen HT, Jehle JA (2009) Stability of Cry1Ab protein during long-term storage for standardization of insect bioassays. Environ Biosaf Res 8:113–119
Anderson PL, Hellmich RL, Prasifka JR, Lewis LC (2005) Effects on fitness and behaviour of monarch butterfly larvae exposed to a combination of Cry1Ab expressing corn anthers and pollen. Environ Entomol 34:944–952
Mattila HR, Sears MK, Duan JJ (2005) Response of Danaus plexippus to pollen of two new Bt corn events via laboratory bioassay. Ent Exp Appl 116:31–41
Zanga D, Sanahuja G, Eizaguirre M, Albajes R, Christou P, Capell T et al (2018) Carotenoids moderate the effectiveness of a Bt gene against the European corn borer, Ostrinia nubilalis. PLOS ONE 13:e0199317
Broderick NA, Raffa KF, Handelsman J (2006) Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc Nat Acad Sci USA 103:15196–15199
Hilbeck A, Defarge N, Bøhn T, Krautter M, Conradin C et al (2018) Impact of antibiotics on efficacy of Cry toxins produced in two different genetically modified Bt maize varieties in two Lepidopteran herbivore species, Ostrinia nubilalis and Spodoptera littoralis. Toxins 10(480):1–17
Hilbeck A, Weiss G, Oehen B, Römbke J, Jänsch S, Teichmann H, Lang A, Otto M, Tappeser B (2014) Ranking matrices as operational tools for the environmental risk assessment of genetically modified crops on non-target organisms. Ecol Ind 36:367–381
Lang A, Ludy C, Vojtech E (2004) Dispersion and deposition of Bt maize pollen in field margins. J Plant Dis Protect 111:417–428
Treu RJ, Emberlin J (2000) Pollen dispersal in the crops maize (Zea mays), oilseed rape (Brassica napus spp. olifera), potatoes (Solanum tuberosum), sugar beet (Beta vulgaris ssp. vulgaris) and wheat (Triticum aestivum). Report for the Soil Association. National Pollen Research Unit, University College, Worcester, UK
Hofmann F, Schlechtriemen U, Kuhn U, Wittich K-P, Koch W et al (2013) Variation of maize pollen shedding in North Germany and its relevance for GMO-monitoring. Theorie in der Ökologie 17:19–25
Andow DA, Hilbeck A (2004) Science-based risk assessment for non-target effects of transgenic crops. Bioscience 54:637–649
Andow D, Zwahlen C (2006) Assessing environmental risks of transgenic plants. Ecol Lett 9:196–214
EFSA European Food Safety Authority (2010) Scientific opinion on statistical considerations for the safety evaluation of GMOs. EFSA J 8(1):1250
Andow DA, Lövei GL, Arpaia S, Wilson L, Fontes EMG, Hilbeck A, Lang A, Van Tuat N, Pires CSS, Sujii ER, Zwahlen C, Birch ANE, Capalbo DMF, Prescott K, Omoto C, Zeilinger AR (2013) An ecologically-based method for selecting ecological indicators for assessing risks to biological diversity from genetically-engineered plants. J Biosaf 22:141–146
EFSA European Food Safety Authority (2014) Guidance on statistical reporting. EFSA J 12(12):3908
Hoenig JM, Heisley DM (2001) The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat 55:19–24
Love M, Latham JR, Hilbeck A (2017) The distinct properties of natural and GM cry insecticidal proteins. Biotechnol Genet Eng Rev 33:62–96
Arpaia S, Birch NE, Kiss J, van Loon JJA, Messéan A, Nuti M, Perry JN, Sweet JB, Tebbe CC (2017) Assessing environmental impacts of genetically modified plants on non-target organisms: the relevance of in planta studies. Sci Total Environ 583:123–132
Acknowledgements
We thank two anonymous reviewers for valuable comments on an earlier version of the manuscript.
Funding
This article has been prepared as an outcome of the BfN research project “Basisdaten zur Effektbewertung verschiedener Bt-Toxine auf Schmetterlingslarven” (FKZ 3515890100).
Author information
Authors and Affiliations
Contributions
MO initiated the study. AL has been responsible for the concept of the study and drafted the manuscript. AL and MO analysed the studies for Bt maize testing. JB reviewed and summarised the NTO testing for chemical plant protection products. The final recommendations for standardising laboratory tests were developed and concluded by all authors. All authors contributed to supporting the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
Overview and methodology of laboratory studies (tier1) submitted in EU market applications to test effects of Cry proteins on Lepidopteran larvae.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lang, A., Lee, M., Dolek, M. et al. Laboratory tests with Lepidoptera to assess non-target effects of Bt maize pollen: analysis of current studies and recommendations for a standardised design. Environ Sci Eur 31, 39 (2019). https://doi.org/10.1186/s12302-019-0220-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-019-0220-2