Abstract
Background
To date, only 8.2% of German surface waters achieve a good ecological status according to the European Water Framework Directive. This is primarily attributed to structural deficits, intensive land use, and chemical contaminations of water bodies. In this context, hydromorphological restoration measures are implemented with the aim to increase habitat and species diversity and thus improve the ecological status of water bodies. Nevertheless, existing studies show that restorations promote the reintroduction of individual species, but only in exceptional cases an improvement in the ecological status is achieved. Therefore, we examined the impact of the prevailing chemical contamination on the restoration success in the catchment of the river Nidda in Hessen (Germany) by comparing restored river sections at the rivers Nidda and Horloff with unrestored sections upstream (space-for-time-substitution) and a transect downstream the restoration measures. For this purpose, we conducted active biomonitoring campaigns with Potamopyrgus antipodarum and Gammarus fossarum and analyzed water and sediment samples with effect-based in vitro bioassays.
Results
At the river Horloff, mortality of P. antipodarum and toxicity in water samples measured via the microtox assay were highest within the restoration. At the river Nidda, the reproduction of snails and gammarids significantly increased within the restorations, and reproduction of snails correlated positively and significantly with estrogenic activities. The microtox assay also exhibited the highest toxicities in water and sediment samples from the restorations and dioxin-like as well as estrogenic activities significantly increased compared to the unrestored reference site. On the basis of these results, the prevailing chemical contamination has negatively affected snails and gammarids in the active biomonitoring campaigns and consequently is likely to have also a negative impact on the local invertebrate community and thus endangers the restoration success.
Conclusion
Hydromorphological restorations as a stand-alone measure are insufficient to improve the ecological status of a water body as long as the water and sediment quality remain deficient. Therefore, it is necessary to improve water and sediment quality in parallel with hydromorphological restoration measures to achieve the objectives of the EU-WFD.
Similar content being viewed by others
Background
For decades, water bodies have been structurally degraded to protect against flooding, gain land for agriculture and settlements, and navigate rivers [1]. To achieve this, rivers were straightened (i.e., channelized) [2, 3], their beds obstructed and monotonous transversal and longitudinal profiles were created to avoid bank erosion and enable navigation [2, 3]. Additionally, wooded banks were cleared [3], gravel banks dredged, river connectivity was hindered by the construction of weirs, and old arms were cut off [4]. This resulted in a loss of aquatic habitats and thus in a decreased biodiversity [2, 5,6,7]. With the European Water Framework Directive (2000/60/EC; EU-WFD) [8], the European Parliament and the Council of the European Union adopted in 2000 a regulatory framework for measures in the field of water policy. The aim of the EU-WFD was to achieve a good ecological and chemical status for all European surface waters by 2015, which is defined by a near-natural water type-specific species assemblage of aquatic flora, benthic invertebrate and fish fauna as well as specific pollutants, hydromorphological, chemical and physico-chemical components as expected in conditions of minimal anthropogenic changes and disturbances [8,9,10]. The ecological status of a given water body is then determined by the worst rated component (“one-out, all-out-principle”) [8]. Accordingly, all stressors, including pollutants, can lead to a loss of species and thus to altered biocenoses, resulting in an inadequate status of water bodies according to the EU-WFD. In principle, it is therefore to be expected that chemical contamination may lead to ecological deficits in water bodies according to the EU-WFD.
In fact, only 8.2% of German surface waters and a minority of rivers in other European countries achieved the required good ecological status according to the EU-WFD by 2015 [9, 10]. This is mainly attributed to structural deficits (e.g., straightening, damming, embankments) [2, 11, 12] and also to chemical contaminations of water bodies (e.g., by wastewater treatment plants, intensive agriculture) [6, 13,14,15,16]. However, the relative contribution of chemical contamination in relation to structural deficits to the inadequate ecological status of water bodies remains unclear.
Nevertheless, hydromorphological restorations with the aim of increasing habitat and species diversity are considered as a key measure to improve the ecological status of water bodies [5, 13, 17, 18]. Such morphological restorations include dismantling of bank and bed fixations [5, 19, 20], removal of weirs to restore river connectivity enabling the migration of fish and invertebrates [20, 21], purchase of land along the watercourse and thus the extensification of land use [12, 20], channel reconfiguration and reconnection of floodplains for flood protection [2, 20], alteration of structural complexity to increase habitat and species diversity [20], as well as the creation of riparian strips to reduce trophic effects by capturing nutrients and toxicants, moderating temperatures and introducing organic matter [2, 21, 22].
However, a number of studies have investigated the efficiency of restoration measures with conflicting results. While few studies reported an enhanced water quality [23,24,25] and an improved diversity of the benthic invertebrate assemblage [17, 26,27,28], others found little signs of improvement even years after the restoration [13, 29,30,31,32,33]. As a possible cause for the poor success of many restoration efforts, the potential impact of the prevailing chemical contamination at two model restorations in the catchment of the river Nidda in Hessen (Germany) is in focus of the present study. For this purpose, we investigated the biological effects caused by chemical contamination in active biomonitoring campaigns, as they represent temporally integrated exposures to pollutants and thus offer a more holistic approach than the investigation of individual pollutants in grab samples, which only provide a snapshot of the chemical contamination in water bodies.
Therefore, we performed active biomonitoring campaigns and laboratory experiments with combined water/sediment samples of the corresponding sampling sites with the freshwater mudsnail Potamopyrgus antipodarum and the amphipod Gammarus fossarum at sites in restored river sections, at unrestored reference sites upstream (space-for-time-substitution [16, 34]) and at a transect downstream the restored sections to account for changes in biological responses. In addition, water and sediment samples from every sampling site were analyzed with effect-based in vitro bioassays (yeast reporter gene assays and microtox assay) to support the in vivo findings.
Methods
Sampling sites and restoration measures
The Nidda catchment, which covers almost 2000 km2, is characterized by intensive agricultural and industrial use [35] and represents a typical catchment of Central Europe. Many water bodies in the catchment have been structurally degraded due to river engineering for flood protection and exhibit numerous obstacles to migration as for example weirs, dams and hydropower plants. In addition, the river Nidda and its tributary Horloff are negatively impacted by intensive agriculture, as well as municipal and industrial wastewater treatment plants (WWTPs) resulting in proportions of clearwater (i.e., treated wastewater) of up to 50% at mean low discharge (MNQ) [36] and thus a potential high chemical contamination level. On the other hand, for more than 20 years restoration measures have been conducted in the Nidda catchment. Despite these efforts, the ecological status of the water bodies has not improved and is still deficient [35, 37]. For this reason, we examined two restoration measures with different intervention depths in a comparative manner: the lower Horloff, whose status in the investigated river sections was assessed with ecological status class 5 (bad), and the river Nidda, which corresponds to ecological status class 4 (poor) throughout the examined sections [35, 37].
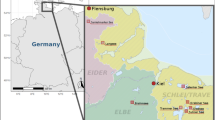
At the river Horloff, we chose three unrestored reference sites upstream of the restored section (marked in white in Fig. 1a) based on space-for-time-substitution [16, 34]. Thus, the reference sites represent the unrestored condition in the same river stretch, which is also subject to the same influences as the restoration. Reference site H1 is located 60 m upstream of a WWTP effluent with a capacity of 78,000 population equivalents (PE) [38], H2 is 160 m downstream the WWTP effluent and H3R, which serves as statistical reference, is about 700 m upstream the restoration site H4. Additionally, the river Horloff receives the surface runoff of the A45 motorway bridge upstream of site H4 [35]. Restoration site H4 is located within a river section which was restored in 2002/2003 and 2006/2007 and extends over a total length of 1.6 km (marked in light grey in Fig. 1a). Due to intensive agriculture, the intervention depth for the restoration measure was lower compared to the second model restoration at the river Nidda. Then five transect sites follow 1.4 km (H5), 2.4 km (H6), 4.3 km (H7), 6.1 km (H8) and 8.1 km (H9) downstream of the restored section, in which possible changes in biological effects were investigated (marked in dark grey in Fig. 1a).
Sampling sites at the river Horloff (a) and sampling sites at the river Nidda (b). Black: wastewater treatment plant, white: reference sites, with H3R and N2R as statistical reference sites, light grey: sites in restored section, dark grey: transect sites. Maps were modified with Adobe® Photoshop CC (Version 20.0.0, Adobe Systems Incorporated, San José, California, US) and are based on kompass.de [39]
At the river Nidda, we selected two unrestored reference sites (marked in white in Fig. 1b) on the basis of space-for-time-substitution [16, 34]. N1 is located 50 m upstream the effluent of a WWTP with a capacity of 48,000 PE [38] and N2R, which serves as statistical reference, lies 500 m downstream the WWTP effluent. Subsequently, three restoration sites N3, N4 and N5 follow (marked in light grey in Fig. 1b). N3 represents the restoration at the so-called “Nidda-Knie” from 2001 and sampling sites N4 and N5 are located within the restoration “Gronauer Hof” from 2010, which extends over a length of 3.2 km. Then four transect sites follow 1.0 km (N6), 1.7 km (N7), 3.2 km (N8) and 6.0 km (N9) downstream the restored section (marked in dark grey in Fig. 1b).
Test organisms
Potamopyrgus antipodarum, the New Zealand mudsnail, was chosen as test organism, as it is a standard organism in the testing of chemicals according to OECD guideline 242 [40] and reacts sensitively towards reproductive toxicants including endocrine disrupting chemicals (EDCs) [41,42,43]. Besides, P. antipodarum has successfully been used in field studies to evaluate the conditions of rivers and environmental samples [44,45,46,47,48]. The snails used in the present study originated from the in-house breeding stock of the Department Aquatic Ecotoxicology at Goethe University, which was kept according to the recommendations of the OECD guideline 242, annex 2 [40].
The genus Gammarus, which includes the freshwater amphipod Gammarus fossarum, reacts sensitively to pollutants such as pesticides (e.g., terbutryn, fenoxycarb) or micropollutants from wastewater (e.g., 17α-ethinylestradiol) [49,50,51,52,53,54,55]. Furthermore, G. fossarum has already been used in field studies to assess the conditions of rivers and environmental samples [56,57,58,59,60]. The gammarids used in the present study were collected 1 day prior to the active biomonitoring campaigns and laboratory experiments from the source region of the river Nidder (N 50°29′7″, E 9°14′52″, Sichenhausen, Hessen, Germany), and kept in aerated river water in a climate chamber at 10 ± 1.5 °C over-night until tests started.
Active biomonitoring campaigns
The active biomonitoring campaigns at the rivers Horloff and Nidda were conducted in March and May 2017, respectively, and were performed as previously described in detail [48], but with minor modifications. Thus, the number of snails and gammarids used in the active biomonitoring campaigns was increased to ten gammarids and ten snails per replicate. The sexing of gammarids prior to the exposure was not possible, since determination of sexes requires a fixation of the individuals. Gammarids with a minimum size of 6.0 mm were introduced in stainless steel enclosures (12.5 cm × 6 cm) with a piece of wire gauze (polytetrafluorethylene, 8.2 cm × 3.3 cm) and conditioned black alder leaves (Alnus glutinosa) ad libitum. Snails with a size of 3.5 to 4.5 mm were introduced in stainless steel tea-eggs (4.5 cm × 3.5 cm) that contained pieces of carrots from controlled biological cultivation ad libitum. At each site, two cages each containing three replicates were exposed (in total 60 snails and 60 gammarids per site). In addition, a data logger (HOBO Pendant®, Onset Computer Corporation, Bourne, USA) was exposed simultaneously at each site measuring the water temperature every 30 min. After 28 days of exposure, snails and gammarids were recovered and checked for mortality. Snails were shock frozen in liquid nitrogen per replicate, and gammarids were fixed separately in 70% ethanol. As endpoints the size and the number of embryos in the brood pouch of snails as a reproductive parameter were measured according to OECD guideline 242 [40], while in gammarids the size, sex, and fecundity index describing the number of eggs depending on the size of the respective gammarid were assessed.
Associated water parameters (water temperature, pH, conductivity, oxygen concentration and saturation) were measured with a portable multimeter (HQ40d, Hach, Germany), ammonium, nitrite, nitrate, ortho-phosphate, sulfate, chloride and dissolved organic carbon (DOC) concentrations were determined via Spectroquant test kits (Merck, Darmstadt, Germany), and total hardness as well as carbonate hardness were determined with MColortest kits (Merck, Darmstadt, Germany) at the beginning of the biomonitoring campaigns.
Laboratory experiments with combined water/sediment samples
Static laboratory experiments with P. antipodarum und G. fossarum using combined water/sediment samples from each site served for plausibility verification to exclude environmental stressors such as water temperature, hydraulic pressure or stream velocity as causes for occurring in vivo effects and were conducted in parallel to the active biomonitoring campaigns. Tests with mudsnails were conducted at 16 ± 1.5 °C in 500 mL glass beakers with 400 mL river water and 40 g sediment according to Duft et al. [41]. The negative control contained 400 mL test medium according to the OECD guideline 242 [40] and 40 g artificial sediment [95% (dw) quartz sand, 5% (dw) powdered beech leaves (Fagus sylvatica)] according to Duft et al. [41]. The test conditions followed the OECD guideline 242 [40] and every combined water/sediment sample and the control were tested in duplicate, each containing 26 individuals of P. antipodarum with sizes between 3.5 and 4.5 mm. Snails were fed three times per week with 70 µg finely ground TetraPhyll® (Tetra GmbH, Melle, Germany) per snail and day.
The tests with gammarids were performed at 10 ± 1.5 °C in 250-mL glass beakers with 200 mL river water and 50 g sediment. The negative control contained 200 mL ISO test water [61] and 50 g artificial sediment according to Duft et al. [41]. In the experiments with gammarids, we used six replicates for the control and four replicates for every combined water/sediment sample, each containing 10 individuals of G. fossarum with a minimum size of 6.0 mm. Gammarids were fed ad libitum with conditioned black alder leaves (Alnus glutinosa). After 28 days of exposure, snails and gammarids were checked for mortality, snails were shock frozen in liquid nitrogen per replicate and gammarids were fixed separately in 70% ethanol and subsequently examined regarding the same endpoints as described for the active biomonitoring campaigns. Associated water parameters (water temperature, pH, conductivity, oxygen saturation and concentration) were measured once per week.
In vitro analyses of water samples
At the start day of the active biomonitoring campaigns, aqueous grab samples were collected at every site. Within 48 h after collection, anti-estrogenic and anti-androgenic activities of unfiltered native water samples were analyzed in the yeast anti-estrogen screen (YAES) and the yeast anti-androgen screen (YAAS) [62]. Thereby, YAES and YAAS require background concentrations of 0.3 nmol 17β-estradiol/L and 10 nmol testosterone/L, respectively.
For the analysis with agonist screens and the microtox assay, water samples were solid-phase extracted (SPE) according to Giebner et al. [62]. Therefore, 1000 mL of each water sample were filtered within 24 h after collection through glass microfibers filters (VWR International GmbH, No. 692, European Cat. No. 516-0885, 90 mm, particle retention: 1.0 µm, Darmstadt, Germany), and the filtrate was passed through conditioned Oasis HLB cartridges (200 mg, Waters, Milford, MA, USA) to capture mid-polar and non-polar substances [63]. Furthermore, a SPE blank was prepared by passing 1000 mL ultrapure water through conditioned cartridges. The cartridges were dried under a gentle stream of nitrogen and eluted with 4 mL methyl tert-butyl ether (MTBE) and 4 mL methanol (MeOH) according to Giebner et al. [62]. Afterwards, 0.1 mL dimethyl sulfoxide (DMSO) was added and the extracts were concentrated under a gentle stream of nitrogen to the final volume of 0.1 mL, which corresponds to a 10,000-fold enrichment. Subsequently, the extracts were analyzed in the yeast estrogen screen (YES), the yeast androgen screen (YAS) [62] and the yeast dioxin screen (YDS) [64]. The maximum DMSO concentration amounted to 0.21% in yeast assays. The measured activities were expressed as equivalent concentrations for 17β-estradiol (YES), testosterone (YAS), 4-hydroxytamoxifen (YAES), flutamide (YAAS) and β-naphthoflavone (YDS) and have been corrected for dilution and enrichment so that equivalent concentrations refer back to native water samples.
In addition, the microtox assay with Aliivibrio fischeri was conducted with water extracts [49, 65]. The maximum DMSO concentration amounted to 1% in the microtox assay. Therefore, the luminescence inhibition in A. fischeri is measured, which is expressed as 50% effect concentration (EC50) referring to the relative enrichment factor (REF) of the respective water sample. An EC50-threshold value of 750 REF was defined for water samples that reached less than 20% luminescence inhibition according to Harth et al. [49]. This threshold is equivalent to the lowest EC50 that a non-toxic sample can reach.
In vitro analyses of sediment samples
Sediment samples were collected from the first two centimeters of the upper sediment layer at each site on the start day of the active biomonitoring campaigns. Sediment samples were freeze-dried (Martin Christ Gefriertrocknungsanlagen GmbH, Alpha 1-4 LSC plus, Osterode, Germany), and 50 g of each sediment sample was shaken with 100 mL ultrapure water at 210 rpm for 10 min (GFL 3017, GFL Gesellschaft für Labortechnik mbH, Burgwedel, Germany), eluted by sonication for 10 min (Sonorex RK 52 H, Bandelin electronic, Berlin, Germany) and afterwards centrifuged at 4400 rpm for 5 min (Centrifuge 5702, Eppendorf AG, Hamburg, Germany). After centrifugation, the estrogenic (YES), androgenic (YAS), anti-estrogenic (YAES), anti-androgenic (YAAS) and the dioxin-like activities (YDS) of the aqueous eluates were measured within 48 h [62, 64]. These activities were expressed as equivalent concentrations per kg sediment for 17β-estradiol (YES), testosterone (YAS), 4-hydroxytamoxifen (YAES), flutamide (YAAS) and β-naphthoflavone (YDS) and corrected regarding dilution.
To quantify the baseline toxicity of sediment samples in the microtox assay with A. fischeri, sediment extracts were prepared. Therefore, 20 g of each freeze-dried sediment sample was extracted with 400 mL acetone in a Soxhlet extractor (Electrothermal EME30500/CEB, Cole-Parmer Ltd., Staffordshire, UK; VWR RC-10 Digital Chiller, VWR International GmbH, Darmstadt, Germany) at 56 °C for 24 h. Sediment extracts were concentrated in a rotary evaporator (Heidolph Laborota 4000-efficient, vacubrand CVC 2000, Heidolph Instruments GmbH & Co. KG, Schwabach, Germany; VWR RC-10 Digital Chiller, VWR International GmbH, Darmstadt, Germany) at 56 °C, 0.5 mL DMSO were added and extracts were reduced under a gentle stream of nitrogen to the final volume of 0.5 mL. These extracts were subsequently analyzed in the microtox assay [49, 65]. The inhibitions of the luminescence are expressed as EC50 referring to mg sediment-equivalents. An EC50 threshold value of 30 mg sediment-equivalents was defined for non-toxic sediment samples, i.e., samples that reached less than 20% inhibition of luminescence. This threshold is equivalent to the lowest EC50 that a non-toxic sample can reach.
In addition, the mean grain size [66] and the loss on ignition [67] were determined in sediment samples from each sampling site.
Data analysis
Statistical analyses were conducted with the software Microsoft® Excel 2016 (Microsoft Corporation, Redmond, USA) and GraphPad Prism®, v.5.04 (GraphPad Software Inc., San Diego, CA, USA). Differences in mortality compared to the corresponding reference site were determined using Fisher’s exact test. Continuous data were examined for normal distribution with D’Agostino and Pearson omnibus normality test and for variance homogeneity with Bartlett’s test for equal variances. In case of normal distribution and variance homogeneity an unpaired t test or a one-way ANOVA with Bonferroni’s post hoc test was applied. If continuous data were not normally distributed or variances were inhomogeneous, a Mann–Whitney test or Kruskal–Wallis test followed by Dunn’s post hoc test was applied. The level of significance was defined as α < 0.05 and is illustrated in the graphs with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). For the correlation of explanatory variables (in vitro activity and baseline toxicity in water and sediment samples, loss on ignition, mean grain size) and the response variables (mortality and reproduction of P. antipodarum and G. fossarum), linear regression analyses were conducted.
Results
Active biomonitoring campaigns and laboratory experiments with Potamopyrgus antipodarum
At the river Horloff, the reproduction of snails exposed at site H2 downstream the wastewater discharge slightly increased compared to H1 but was not significantly enhanced in the active biomonitoring campaign (Fig. 2a). At sites H1 and H2 also significantly fewer snails died than at reference site H3R (p < 0.05–0.01, Fig. 2c). At restoration site H4, we found the highest mortality of P. antipodarum among all sites at the Horloff (Fig. 2c). Here, 53.3% of the exposed snails died which corresponds to a significantly higher mortality than at reference site H3R (p < 0.01). At the transect sites, the reproduction varied around the reference level (Fig. 2a), the mortality of P. antipodarum decreased again and was, in case of transect site H8, significantly reduced compared to reference site H3R (p < 0.01, Fig. 2c). A similar trend in the reproduction of P. antipodarum was also observed in laboratory experiments with combined water/sediment samples from the corresponding sampling sites (Additional file 1: Figure S1a).
Potamopyrgus antipodarum. Mean and standard deviation of the number of embryos (a, b) and mean and standard error of the mean of the percentage mortality (c, d) after 28 days of exposure in the active biomonitoring campaigns at the river Horloff in March 2017 (a, c) and the river Nidda in May 2017 (b, d). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in the number of embryos compared to the reference site N2R (shaded) were determined via unpaired t test. Significant differences in the percentage mortality compared to the corresponding reference site H3R or N2R (shaded) were determined using Fisher’s exact test. *p < 0.05, **p < 0.01, ***p < 0.001, n = 6
In contrast to the river Horloff, snails produced considerably more embryos in the active biomonitoring campaign at the river Nidda (cf., Fig. 2a, b). At reference site N2R downstream the wastewater discharge, snails produced slightly less embryos compared to site N1, but this was not statistically significant (Fig. 2b). At the restoration sites N3 to N5, snails’ reproduction was significantly higher than at reference site N2R (p < 0.05–0.01), whereas mortality was not significantly affected (Fig. 2b, d). Reproduction of P. antipodarum corresponded in the transect sites to the reference level at N1 (Fig. 2b) and mortality rose significantly, reaching 33.3% and 31.7% at transect sites N7 and N9, respectively (p < 0.001, Fig. 2d). In laboratory experiments with P. antipodarum and combined water/sediment samples from the river Nidda, we found a different pattern for the reproduction and mortality (Additional file 1: Figure S1b, d). Here, the embryo numbers in snails significantly decreased at restoration site N5 compared to N2R (p < 0.05) and mortality was not affected.
Active biomonitoring campaigns and laboratory experiments with Gammarus fossarum
At the river Horloff, the fecundity indices of gammarids slightly increased from sites H2 to H4 (Fig. 3a), whereas the mortality decreased slightly at these sites compared to H1 (Fig. 3c), but these differences were not statistically significant. At the transect sites H5 (p < 0.001) and H8 (p < 0.05), significantly fewer G. fossarum individuals died, whereas at H7 a significantly higher mortality of gammarids was observed compared to H3R (p < 0.01, Fig. 3c). At H9, the highest fecundity index was recorded but did not differ significantly from H3R (Fig. 3a). Also, in the laboratory experiment with combined water/sediment samples from the river Horloff, the fecundity index did not differ significantly between sampling sites (Additional file 2: Figure S2a). In contrast to the active biomonitoring at the river Horloff, significantly fewer gammarids died at site H1 in the laboratory experiment than at reference site H3R (p < 0.05, Additional file 2: Figure S2c). The highest mortality with 25% dead G. fossarum individuals was observed at the reference site H3R in laboratory experiments, and a significantly lower mortality was determined at the restoration site H4 (p < 0.01, Additional file 2: Figure S2c).
Gammarus fossarum. Mean and standard deviation of the fecundity index (a, b) and mean and standard error of the mean of the percentage mortality (c, d) after 28 days of exposure in the active biomonitoring campaigns at the river Horloff in March 2017 (a, c) and the river Nidda in May 2017 (b, d). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in the fecundity index compared to the reference site N2R (shaded) were determined via unpaired t test. No brooding females occurred at N7 and N9, therefore, fecundity index could not be calculated. Significant differences in the percentage mortality compared to the corresponding reference site H3R or N2R (shaded) were determined using Fisher’s exact tests. *p < 0.05, **p < 0.01, ***p < 0.001, n = 6
At the river Nidda, the fecundity index of gammarids declined by 43% at reference site N2R downstream the wastewater discharge, whereas mortality rose by 78% compared to site N1 (Fig. 3b, d), but these differences were not statistically significant. At the restoration site N3, the fecundity index significantly increased compared to the reference site N2R (p < 0.01, Fig. 3b) and the mortality remained on a comparable level as at site N2R (Fig. 3d). At N5, the fecundity index was highest among all sites at the river Nidda (Fig. 3b) but was not statistically significant compared to N2R due to the high standard deviation. At the transect site N6, the fecundity index was significantly higher than at reference site N2R (p < 0.05, Fig. 3b) and at N7 and N9, no reproduction of gammarids occurred (Fig. 3b), since the mortality increased significantly to 76.7% and 83.3%, respectively (p < 0.001, Fig. 3d). In the laboratory experiment with combined water/sediment samples from the river Nidda, completely different patterns were observed for fecundity and mortality (Additional file 2: Figure S2b, d). Within the restoration sites, the fecundity indices showed a decreasing trend compared to reference site N2R and reached the lowest fecundity index at N5. Significantly fewer gammarids died at reference site N1 (p < 0.05), restoration site N3 (p < 0.001) as well as transect sites N6 (p < 0.001), N8 (p < 0.05) and N9 (p < 0.001) compared to N2R.
In vitro analyses of water samples
The microtox assay revealed already moderately toxic water samples at the Horloff reference sites H1 to H3R (Fig. 4a). At sites H1 and H2, we also found significantly increased anti-estrogenic activities with 2.21 and 1.48 mg OHT-EQ/L, respectively, compared to H3R (p < 0.01–0.001, Additional file 3: Figure S3a). Furthermore, we also found slight dioxin-like activities in water samples from sites H2 and H3R (Fig. 4c), but these were very low and close to the detection limit (Additional file 4: Table S4). Surprisingly, the baseline toxicity at restoration site H4 increased significantly by 66% compared to the reference site H3R (p < 0.05) and, therefore, represented the most toxic water sample from the river Horloff (Fig. 4a). The baseline toxicity of water samples declined within the transect sites compared to H3R, reaching a significantly increased EC50 of 750 REF at H6 and H7, which is equivalent to non-toxic water samples (p < 0.001, Fig. 4a). At site H7, we also found significantly increased dioxin-like and anti-estrogenic activities with 0.495 mg OHT-EQ/L compared to H3R (p < 0.01, Fig. 4c, Additional file 3: Figure S3a). However, these activities were comparatively low and close to the detection limits (Additional file 4: Table S4). In water samples from the river Horloff, no estrogenic activities were found at any sampling site (Fig. 4e).
Mean and standard error of the mean of EC50 for baseline toxicity (a, b), dioxin-like activity (c, d) and estrogenic activity (e, f) in water samples from the river Horloff in March 2017 (a, c, e) and the river Nidda in May 2017 (b, d, f). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in baseline toxicity compared to the reference site H3R (shaded) were determined using one-way ANOVA and Bonferroni’s post hoc test. Significant differences in dioxin-like activity and estrogenic activity compared to the corresponding reference site H3R or N2R (shaded) were determined via Kruskal–Wallis test with Dunn’s post hoc test. If no bar is illustrated, the activity was below the LOQ or no activity at all was measured. *p < 0.05, **p < 0.01, ***p < 0.001, n = 3 with 8 pseudo-replicates each
At the river Nidda, we also found a moderate baseline toxicity in water samples at the reference sites N1 and N2R as well as the highest baseline toxicities in water samples from the restoration sites N3 to N5 (Fig. 4b), but these did not differ significantly from N2R. At these restoration sites, the dioxin-like activities significantly raised by up to 92% (p < 0.001, Fig. 4d) and the estrogenic activities significantly increased by up to 124% compared to N2R (p < 0.001, Fig. 4f). The baseline toxicities of water samples from transect sites N6 and N8 reached a comparable level as at the restoration sites N3 to N5 (Fig. 4b). At these transect sites, the dioxin-like (Fig. 4d) and the estrogenic activities (Fig. 4f) were also significantly higher compared to N2R (p < 0.05–0.001). Also, the anti-estrogenic activity was at N8 with 24.6 mg OHT-EQ/L considerably higher but not significantly different from reference site N2R (14 mg OHT-EQ/L) and increased significantly at N9 with 28.8 mg OHT-EQ/L (p < 0.05, Additional file 3: Figure S3b).
In vitro analyses of sediment samples
The microtox assay revealed at site H2 downstream the wastewater discharger the highest baseline toxicity of sediment samples from the river Horloff (Fig. 5a) but did not differ significantly from H3R. At this site, we also observed an increasing trend in the dioxin-like activity compared to H1 (Fig. 5c). At the restoration site H4 and the transect sites, baseline toxicity and dioxin-like activity varied around the reference level of H3R.
Mean and standard error of the mean of EC50 for baseline toxicity (a, b) and dioxin-like activity (c, d) in sediment samples from the river Horloff in March 2017 (a, c) and the river Nidda in May 2017 (b, d). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in baseline toxicity compared to the reference site N2R (shaded) were determined using one-way ANOVA and Bonferroni’s post hoc test. Significant differences in dioxin-like activity compared to reference site N2R (shaded) were determined via Kruskal–Wallis test with Dunn’s post hoc test. If no bar is illustrated, the activity was below the LOQ or no activity at all was measured. **p < 0.01, ***p < 0.001, n = 3 with 8 pseudo-replicates each
In the microtox assay with sediment samples from the river Nidda, the highest baseline toxicity was found at restoration site N3 but did not differ significantly from reference site N2R (Fig. 5b). At reference site N2R and restoration sites N3 and N4 no dioxin-like activities were found, since they were below the LOQ (Fig. 5d, Additional file 4: Table S4). The least toxic sediment from the river Nidda and, thus, a significantly higher EC50 were determined at N7 (p < 0.01, Fig. 5b). At this site, we found a significantly increased dioxin-like activity compared to reference site N2R (p < 0.001, Fig. 5d). However, the activity was comparably low and close to the detection limit (Additional file 4: Table S4). At N9, the dioxin-like activity significantly rose to 38.5 µg β-NF-EQ/kg (p < 0.01, Fig. 5d).
Anti-estrogenic activities were only found in sediments from reference site N1 and the restoration site N3 with 152 mg OHT-EQ/kg and 84.8 mg OHT-EQ/kg, respectively.
The results of the mean grain size of sediment samples from the river Horloff revealed, that nearly all sampling sites, except restoration site H4 and transect site H9, belong to the sediment category fine sand (Table 1). The highest loss on ignition and, thus, the highest organic content was found at reference site H2 and at transect site H6.
At the river Nidda, the mean grain size of most sediment samples lay between 0.2 and 0.6 mm, which corresponds to sediment category medium sand. Only reference site N2R and restoration site N3 exhibited mean grain sizes ranging from 0.06 to 0.2 mm and correspond to sediment category fine sand. The highest loss on ignition was measured in sediment samples from the reference site N2R, the restoration site N5 and the transect site N8.
Linear correlation analyses
The results of the linear correlation analyses between explanatory variables (endocrine activity and toxicity of water and sediment samples, loss on ignition, mean grain size) and response variables (reproduction and mortality of P. antipodarum and G. fossarum) are summarized in Table 2 and are illustrated in Additional file 5: Figure S4. In addition to the linear correlations of Table 2, we found a significant positive correlation between the estrogenic and the dioxin-like activity in water samples (p < 0.001, Additional file 5: Figure S4i) as well as a significant negative correlation between the loss on ignition and the dioxin-like activity in water samples (p < 0.05, Additional file 5: Figure S4f).
Discussion
Horloff
Since reproduction of snails and gammarids increased or at least showed an increasing trend at H2 in both, the active biomonitoring campaign and under standardized conditions in the laboratory, this cannot be explained by the higher water temperature (+ 0.78 °C) due to the discharge of the WWTP. However, the organic carbon content in sediments increased considerably from sampling site H1 with 8.45% dry weight (dw) to 14.1% dw at site H2, which is possibly due to the discharge of the WWTP (Table 1). A higher proportion of organic matter may provide additional food for the detritivorous mudsnails and gammarids, probably increasing reproduction [45, 68]. In addition, the enhanced food supply may have masked toxic effects, such as the substantially increased sediment toxicity at H2 compared to H1 (Fig. 5a) [69,70,71,72]. It is also conceivable that the increased organic content in sediments, the smaller mean particle size (Table 1) and the increased DOC (Additional file 4: Table S1) could have bound organic pollutants and thus reduced the bioavailability of toxic substances for snails and gammarids [68, 73,74,75,76,77,78,79]. Therefore, no toxic effects, i.e., no significantly increased mortality of P. antipodarum and G. fossarum, occurred despite the high sediment toxicity determined in the microtox assay at H2 following a total extraction of sediments via Soxhlet (Fig. 5a). This is in line with the observations of Schmitt et al. [80], who found significant increases in the estrogenic activity after total extraction of sediments, while the reproduction of P. antipodarum was not enhanced. Furthermore, the anti-estrogenic activity declined at site H2 compared to H1 (Additional file 3: Figure S3a), which may have also contributed to the higher reproduction at site H2, since anti-estrogens are able to reduce the reproduction of snails [41, 81, 82].
The high mortality of P. antipodarum within the restoration measure in the active biomonitoring cannot be attributed to differences in associated water parameters, since these differed just slightly between sampling sites (Additional file 4: Table S1) and is rather due to the significantly increased baseline toxicity in water (Fig. 4a), the high sediment toxicity (Fig. 5a) and the dioxin-like activity at site H4 (Fig. 5c). The decline in water and sediment quality is likely to result from the surface runoff of the A45 motorway bridge and might be attributed to the presence of polycyclic aromatic hydrocarbons (PAHs) and metals in sediments and water phase [74, 83, 84] which would also explain the in vitro results in the present study. In contrast to the active biomonitoring, no significant increase in the mortality of P. antipodarum occurred in laboratory experiments. This may be due to the use of grab samples of river water and sediments that may have been taken before contaminants appeared and affected snails and gammarids in the active biomonitoring campaign. Moreover, it is conceivable that contaminant concentrations have decreased through chemical and biological degradation since no water renewal was conducted in laboratory experiments.
Although most invertebrates do not express the aryl hydrocarbon receptor (AhR) and are, therefore, relatively unresponsive to dioxin-like compounds [85], PAHs and dioxins as potent agonists at the AhR [85,86,87] negatively affect reproduction and mortality of invertebrates [74, 88,89,90,91,92,93]. The correlation analyses also revealed that increasing dioxin-like activities lead to a decrease in the reproduction of G. fossarum (Table 2, Additional file 5: Figure S4h) but do not have a lethal effect on G. fossarum and P. antipodarum. Therefore, an influence of dioxin-like substances on the reproduction of G. fossarum and P. antipodarum cannot be excluded in the present study and might have contributed to the biological responses.
Nidda
A possible explanation for the decreasing trend in reproduction and the increasing trend in mortality of snails and gammarids downstream the WWTP discharger is the 22% lesser DOC concentration at site N2R compared to N1 (Additional file 4: Table S1) so that less hydrophobic organic contaminants are bound, thus increasing bioavailability and toxicity of these pollutants to aquatic organism [77,78,79, 94].
The results of the river Nidda revealed a substantially higher level of endocrine activity and toxicity in restored river sections compared to the reference site upstream, although not all observed endpoints were significantly elevated. This especially refers to estrogenic and dioxin-like activities in water samples as well as baseline toxicity in water and sediment samples (Figs. 4b, d, f, 5b). The estrogenic activity in water samples correlates significantly and positively with the embryo numbers in P. antipodarum (cf., Figs. 2b, 4f, Table 2, Additional file 5: Figure S4a), for which an increase in reproduction with rising estrogenic activities has already been reported [41, 47, 80, 93, 95]. Since only reduced reproduction and increased mortality of invertebrates by dioxin-like substances are described in the literature [83, 88,89,90,91] but no increased reproduction, it can be assumed that the significant positive correlation between dioxin-like activities and the reproduction of snails only reflects the wastewater load and thus the estrogenic activity (Table 2, Additional file 5: Figure S4b). This results mainly from the correlation between estrogenic and dioxin-like compounds in water samples and is supported by the significant negative correlation between dioxin-like activities in sediment samples and the reproduction of P. antipodarum (Table 2, Additional file 5: Figure S4c, i). Moreover, the correlation analyses showed that the reproduction of snails in the active biomonitoring increases with increasing mean grain size and decreases with increasing loss on ignition (Table 2, Additional file 5: Figure S4d, e). This was to be expected as the bioavailability of estrogens increases with increasing mean grain size and decreases with increasing organic content in sediments [96,97,98], so that these could have contributed indirectly to the elevated reproduction of snails in restored river sections.
In contrast, the relation between water and sediment contamination and the reproduction of G. fossarum is less clear. On the one hand, Schneider et al. [53] observed an increase in the fecundity index of Gammarus pulex with increasing wastewater content and attributed this to the presence of EDCs, especially to estrogenic substances, which is in line with the present findings within restored river sections in the active biomonitoring; on the other hand, various studies report on decreasing fecundity indices downstream of WWTP effluents or on shifts in sex ratio in favor of females induced by estrogens but not on elevated fecundity of gammarids [50, 53, 54]. Thus, a reliable proof of an increased fecundity of G. fossarum due to estrogen exposure is not yet available and correlation analyses in the present study revealed decreasing fecundity indices with increasing estrogenic or dioxin-like activity, which is likely due to the correlation between estrogenic and dioxin-like activity in water samples (Table 2, Additional file 5: Figure S4g, i). Therefore, it is assumed that estrogenic activity represents just the wastewater load and that dioxin-like activities are responsible for a decrease in fecundity indices. However, the cause for an increased reproduction of gammarids within restored river sections remains unknown in the present study.
The effect pattern on reproductive parameters in the laboratory experiments with P. antipodarum and G. fossarum showed a completely different picture (Additional file 1: Figure S1b, Additional file 2: Figure S2b), probably due to a superimposition of estrogenic and toxic effects (cf., Figs. 4b, d, f, 5b). Snails and gammarids in the active biomonitoring campaigns are primarily exposed via the water phase, while they are more affected in laboratory experiments by substances in the sediment. As already mentioned, the biomonitoring campaigns represent temporally integrated exposures to substances in the water phase, whereas laboratory experiments were performed with grab samples of water and sediments and, therefore, only represent snapshots of the chemical contamination at a certain time. Estrogenic substances in water samples may have been degraded during the 28-day exposure in laboratory experiments as stated in Schneider et al. [53], who showed a halving of estrogenic activity within 24 h, whereas the contamination of sediments remains comparably stable. Therefore, an increased reproduction as a consequence of estrogen exposure was not observed in laboratory experiments with snails and gammarids but reduced reproduction, probably due to the baseline toxicity in sediments and the dioxin-like activities in water and sediment samples known to be highly resistant to chemical and biological degradation [99]. Correlation analyses also revealed a decreasing number of embryos in P. antipodarum with increasing dioxin-like activity in sediment samples (Table 2, Additional file 5: Figure S4c).
Comparison of the rivers Horloff and Nidda
The reproduction of mudsnails at the river Nidda was much higher than at the river Horloff, which can partially be explained by the higher water temperature in May 2017 in the Nidda (N1: 18.1 ± 2.07 °C) compared to the Horloff in March 2017 (H1: 9.82 ± 1.97 °C), since reproduction of P. antipodarum is temperature-dependent [100] and snails in the river Nidda were exposed almost to their optimum temperature [101]. Besides the substantially higher water temperature, the significantly higher estrogenic activity in combination with a higher bioavailability due to lower organic carbon contents and higher mean particle sizes (Table 1) [96,97,98] in the Nidda is a likely cause for the higher embryo numbers in P. antipodarum compared to the river Horloff [42, 47, 80, 95]. The higher reproduction at the reference sites of the Nidda compared to the Horloff is likely due to higher water temperature and higher estrogen exposure as measured by the YES. But since the reproduction of snails and the estrogenic activity at the river Nidda highly correlate over the course of all sampling sites (Additional file 5: Figure S4a) and the temperatures are more or less constant, the estrogenic compounds are the likely explanation for the significantly increased embryo numbers within the restoration sites at the river Nidda. In contrast to the snails, gammarids exhibited higher fecundity indices at the river Horloff than at the river Nidda, which is probably due to the lower average water temperature at the Horloff in March compared to the river Nidda in May, considering that the optimum temperature for G. fossarum is 12.1 °C [102, 103]. In addition, a substantially higher contamination with dioxin-like substances could be detected in the Nidda than in the Horloff, which could have also contributed to the lower reproduction of the gammarids in the Nidda (Additional file 5: Figure S4h).
The in vitro assays indicated a higher level of activity and, therefore, contamination in the river Nidda, although the analyzed section was restored with a considerably higher intervention depth and is primarily surrounded by grassland and pastures, while the river Horloff is mainly surrounded by intensive agricultural land. The most probable causes for the increased in vitro activities in river water and sediments are wastewater discharges [104,105,106,107], intensive agricultural use [108,109,110,111] and surface runoff from motorways [74, 83, 84]. Since riverine sediments represent a major sink for some contaminants [96, 97, 112,113,114,115,116,117,118], the lower level of activity at the river Horloff could likely be due to the higher organic content and the smaller mean grain size of sediments so that contaminants are less bioavailable (except for the microtox assay with sediment samples for which a total extraction was performed) [68, 73, 74, 97]. This is also supported by the significant negative correlation between loss on ignition and dioxin-like activity in water samples (Additional file 5: Figure S4f).
As this is the first study to report an increase in toxicity as well as estrogenic and dioxin-like activity in restored river sections, this may explain the lack of success of many other in-stream restoration projects [13, 29, 31,32,33]. A possible explanation for the significantly worse results within the restored sections is the transport and deposition of polluted fine sediments within the restoration measures [18, 119,120,121]. Since the restored sections are characterized by a higher flow diversity, it is conceivable that polluted fine particulate matter, e.g., introduced by soil erosion from surrounding fields [122,123,124,125] or by WWTPs [107, 125], may settle in the flow-calmed zones [119, 126,127,128]. The reduced flow velocity might result in an increased exchange between sediment and water, so that formerly sediment associated substances are remobilized and affect the local invertebrate fauna [96, 97, 122, 129]. Furthermore, remobilization of sediment associated contaminants increases by bioturbating activities of sediment dwelling invertebrates [130,131,132,133] and dredging activities during the restoration process [18, 123, 134, 135]. This underlines that river sediments are both important sinks and sources of contaminants [85, 115, 116, 136].
Hence, the present study shows that the success of restoration measures is endangered by the prevailing chemical contamination, which was assessed via in vivo whole organism and effect-based in vitro bioassays. Thus, restoration measures on their own will not lead to the desired good ecological status according to EU-WFD unless chemical contamination of water and sediments is reduced in parallel. As our approach provides clear advantages compared to the assessment of the chemical status according to EU-WFD, which focuses only on the concentrations of 45 priority substances and completely neglects effects of metabolites, transformation products, non-regulated substitutes of priority substances and mixture effects of substances [137,138,139,140,141,142,143], while these can be assessed by effect-based in vitro bioassays, we recommend implementing effect-based methods in the EU-WFD [137,138,139, 144] and improving water and sediment quality in conjunction with hydromorphological restoration measures to achieve the objectives of the EU-WFD.
Conclusion
The in vivo and in vitro assessments yielded the worst results for the restored sections. In addition, the measured in vitro activities and in vivo effects for the river Nidda were even worse than for the river Horloff, although the Nidda was restored with a considerably higher intervention depth. Accordingly, restoration measures do not seem to have any compensating positive influence on the organisms that are affected by chemical contamination, irrespective of the intervention depth of the restoration. Furthermore, the results revealed that the prevailing chemical contamination negatively affected snails and gammarids in the active biomonitoring campaigns and consequently will also affect the local invertebrate community and thus endangers the restoration success.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- AhR:
-
aryl hydrocarbon receptor
- β-NF-EQ:
-
β-naphthoflavone-equivalents
- 95% CI:
-
95% confidence interval
- DMSO:
-
dimethyl sulfoxide
- DOC:
-
dissolved organic carbon
- dw:
-
dry weight
- EC50 :
-
50% effect concentration
- EDC:
-
endocrine disrupting chemical
- EEQ :
-
17β-estradiol-equivalents
- EU-WFD:
-
European Water Framework Directive
- Flu-EQ:
-
flutamide-equivalents
- LOQ:
-
limit of quantification
- MeOH:
-
methanol
- MNQ:
-
mean low discharge
- MTBE:
-
methyl tert-butyl ether
- OHT-EQ :
-
4-hydroxytamoxifen-equivalents
- PAH:
-
polycyclic aromatic hydrocarbon
- PE:
-
population equivalents
- REF:
-
relative enrichment factor
- SD:
-
standard deviation
- SEM:
-
standard error of the mean
- SPE:
-
solid-phase extraction
- TCDD:
-
2,3,7,8-tetrachlorodibenzo-p-dioxin
- T-EQ:
-
testosterone-equivalents
- WWTP:
-
wastewater treatment plant
- YAAS:
-
yeast anti-androgen screen
- YAES:
-
yeast anti-estrogen screen
- YAS:
-
yeast androgen screen
- YDS:
-
yeast dioxin screen
- YES:
-
yeast estrogen screen
References
Reyjol Y, Argillier C, Bonne W, Borja A, Buijse AD, Cardoso AC, Daufresne M, Kernan M, Ferreira MT, Poikane S, Prat N, Solheim A-L, Stroffek S, Usseglio-Polatera P, Villeneuve B, Van de Bund W (2014) Assessing the ecological status in the context of the European Water Framework Directive: where do we go now? Sci Total Environ 497–498:332–344. https://doi.org/10.1016/j.scitotenv.2014.07.119
Wenger SJ, Roy AH, Jackson CR, Bernhardt ES, Carter TL, Filoso S, Gibson CA, Hession WC, Kaushal SS, Martí E, Meyer JL, Palmer MA, Paul MJ, Purcell AH, Ramírez A, Rosemond AD, Schofield KA, Sudduth EB, Walsh CJ (2009) Twenty-six key research questions in urban stream ecology: an assessment of the state of the science. J N Am Benthol Soc 28(4):1080–1098. https://doi.org/10.1899/08-186.1
Tullos DD, Penrose DL, Jennings GD (2006) Development and application of a bioindicator for benthic habitat enhancement in the North Carolina Piedmont. Ecol Eng 27(3):228–241. https://doi.org/10.1016/j.ecoleng.2006.03.001
Popp H, Lehr G (2008) Renaturierungsprojekte in Hessen am Beispiel der Wisper und der Nidda - Versuch einer Erfolgsbewertung. In: Deutscher Rat für Landespflege (Hrsg.) Kompensation von Strukturdefiziten in Fließgewässern durch Strahlwirkung. Schr-R d Deutschen Rates für Landespflege 81, pp 93–95
Miller SW, Budy P, Schmidt JC (2010) Quantifying macroinvertebrate responses to in-stream habitat restoration: applications of meta-analysis to river restoration. Restor Ecol 18(1):8–19. https://doi.org/10.1111/j.1526-100X.2009.00605.x
Palmer MA, Menninger HL, Bernhardt E (2010) River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshw Biol 55(1):205–222. https://doi.org/10.1111/j.1365-2427.2009.02372.x
Brooker MP (1985) The ecological effects of channelization. Geogr J 151(1):63–69. https://doi.org/10.2307/633280
European Parliament and the European Council (2000) Directive 2000/6/EC of the European Parliament and the Council of 23 October 2000 establishing a framework for Community action in the field of water policy (short: Water Framework Directive)
BMUB & UBA (2016) Die Wasserrahmenrichtlinie-Deutschlands Gewässer 2015, Bonn, Dessau, Deutschland
UBA (2017) Gewässer in Deutschland: Zustand und Bewertung. UBA, Dessau-Roßlau
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24(3):706–723. https://doi.org/10.1899/04-028.1
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst 35(1):257–284. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122
Sundermann A, Antons C, Cron N, Lorenz AW, Hering D, Haase P (2011) Hydromorphological restoration of running waters: effects on benthic invertebrate assemblages. Freshw Biol 56(8):1689–1702. https://doi.org/10.1111/j.1365-2427.2011.02599.x
Sundermann A, Gerhardt M, Kappes H, Haase P (2013) Stressor prioritisation in riverine ecosystems: which environmental factors shape benthic invertebrate assemblage metrics? Ecol Indic 27:83–96. https://doi.org/10.1016/j.ecolind.2012.12.003
Sundermann A, Leps M, Leisner S, Haase P (2015) Taxon-specific physico-chemical change points for stream benthic invertebrates. Ecol Indic 57:314–323. https://doi.org/10.1016/j.ecolind.2015.04.043
Haase P, Hering D, Jähnig SC, Lorenz AW, Sundermann A (2013) The impact of hydromorphological restoration on river ecological status: a comparison of fish, benthic invertebrates, and macrophytes. Hydrobiologia 704(1):475–488. https://doi.org/10.1007/s10750-012-1255-1
Li K, Zhang Z, Yang H, Bian H, Jiang H, Sheng L, He C (2018) Effects of instream restoration measures on the physical habitats and benthic macroinvertebrates in an agricultural headwater stream. Ecol Eng 122:252–262. https://doi.org/10.1016/j.ecoleng.2018.08.007
Pander J, Mueller M, Geist J (2015) A comparison of four stream substratum restoration techniques concerning interstitial conditions and downstream effects. River Res Appl 31(2):239–255. https://doi.org/10.1002/rra.2732
Lepori F, Palm D, Brännäs E, Malmqvist B (2005) Does restoration of structural heterogeneity in streams enhance fish and macroinvertebrate diversity? Ecol Appl 15(6):2060–2071. https://doi.org/10.1890/04-1372
Wohl E, Lane SN, Wilcox AC (2015) The science and practice of river restoration. Water Resour Res 51(8):5974–5997. https://doi.org/10.1002/2014WR016874
LANUV (2011) Strahlwirkungs- und Trittsteinkonzept in der Planungspraxis. LANUV-Arbeitsblatt 16. https://www.lanuv.nrw.de/fileadmin/lanuvpubl/4_arbeitsblaetter/40016.pdf. Accessed 28 Nov 2018
Roni P, Beechie TJ, Bilby RE, Leonetti FE, Pollock MM, Pess GR (2002) A review of stream restoration techniques and a hierarchical strategy for prioritizing restoration in Pacific Northwest watersheds. N Am J Fish Manag 22(1):1–20. https://doi.org/10.1577/1548-8675(2002)022%3c0001:AROSRT%3e2.0.CO;2
Lawrence JE, Skold ME, Hussain FA, Silverman DR, Resh VH, Sedlak DL, Luthy RG, McCray JE (2013) Hyporheic zone in urban streams: a review and opportunities for enhancing water quality and improving aquatic habitat by active management. Environ Eng Sci 30(8):480–501. https://doi.org/10.1089/ees.2012.0235
Roley SS, Tank JL, Williams MA (2012) Hydrologic connectivity increases denitrification in the hyporheic zone and restored floodplains of an agricultural stream. J Geophys Res. https://doi.org/10.1029/2012jg001950
Klocker CA, Kaushal SS, Groffman PM, Mayer PM, Morgan RP (2009) Nitrogen uptake and denitrification in restored and unrestored streams in urban Maryland, USA. Aquat Sci 71(4):411–424. https://doi.org/10.1007/s00027-009-0118-y
Verdonschot RCM, Kail J, McKie BG, Verdonschot PFM (2016) The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 769(1):55–66. https://doi.org/10.1007/s10750-015-2575-8
Kail J, Brabec K, Poppe M, Januschke K (2015) The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: a meta-analysis. Ecol Indic 58:311–321. https://doi.org/10.1016/j.ecolind.2015.06.011
Frainer A, Polvi LE, Jansson R, McKie BG, Cao Y (2018) Enhanced ecosystem functioning following stream restoration: the roles of habitat heterogeneity and invertebrate species traits. J Appl Ecol 55(1):377–385. https://doi.org/10.1111/1365-2664.12932
Friberg N, Baattrup-Pedersen A, Kristensen EA, Kronvang B, Larsen SE, Pedersen ML, Skriver J, Thodsen H, Wiberg-Larsen P (2014) The river Gelså restoration revisited: habitat specific assemblages and persistence of the macroinvertebrate community over an 11-year period. Ecol Eng 66:150–157. https://doi.org/10.1016/j.ecoleng.2013.09.069
Feld CK, Birk S, Bradley DC, Hering D, Kail J, Marzin A, Melcher A, Nemitz D, Pedersen ML, Pletterbauer F, Pont D, Verdonschot PFM, Friberg N (2011) From natural to degraded rivers and back again. Adv Ecol Res 44:119–209. https://doi.org/10.1016/B978-0-12-374794-5.00003-1
Louhi P, Mykrä H, Paavola R, Huusko A, Vehanen T, Mäki-Petäys A, Muotka T (2011) Twenty years of stream restoration in Finland: little response by benthic macroinvertebrate communities. Ecol Appl 21(6):1950–1961. https://doi.org/10.1890/10-0591.1
Jähnig SC, Brabec K, Buffagni A, Erba S, Lorenz AW, Ofenböck T, Verdonschot PFM, Hering D (2010) A comparative analysis of restoration measures and their effects on hydromorphology and benthic invertebrates in 26 central and southern European rivers. J Appl Ecol 47(3):671–680. https://doi.org/10.1111/j.1365-2664.2010.01807.x
Violin CR, Cada P, Sudduth EB, Hassett BA, Penrose DL, Bernhardt ES (2011) Effects of urbanization and urban stream restoration on the physical and biological structure of stream ecosystems. Ecol Appl 21(6):1932–1949. https://doi.org/10.1890/10-1551.1
Hey RD, Heritage GL, Patteson M (1994) Impact of flood alleviation schemes on aquatic macrophytes. Regul River 9:103–119
HLNUG: Hessisches Karteninformationssystem (WRRL-Viewer). http://wrrl.hessen.de. Accessed 15 Aug 2018
HLUG (2004) Jahresbericht zur Durchführung des hessischen Programmes nach § 3 der Qualitätszielverordnung und Artikel 7 der Richtlinie 76/464/EWG. https://www.hlnug.de/fileadmin/dokumente/wasser/fliessgewaesser/gewaesserbelastung/2004.pdf. Accessed 12 Oct 2018
Haase P, Huck S, Kaffenberger N, Korte E, Leps M, Mährlein M, Michl T (2019) Ökologisches Langzeitmonitoring und Erfolgskontrolle für die Renaturierungsmaßnahme an der Nidda bei Bad Vilbel-Dortelweil: 3. Zwischenbericht zum Projekt
HMUKLV (2017) Beseitigung von kommunalen Abwässern in Hessen: Lagebericht 2016, Wiesbaden, Germany
KOMPASS-Karten GmbH: Karteninformationssystem. http://www.kompass.de. Accessed 14 Nov 2018
OECD (2016) Guideline for testing of chemicals: Potamopyrgus antipodarum reproduction test (Guideline 242, adopted 29th July 2016). OECD, Paris
Duft M, Schmitt C, Bachmann J, Brandelik C, Schulte-Oehlmann U, Oehlmann J (2007) Prosobranch snails as test organisms for the assessment of endocrine active chemicals—an overview and a guideline proposal for a reproduction test with the freshwater mudsnail Potamopyrgus antipodarum. Ecotoxicology 16(1):169–182. https://doi.org/10.1007/s10646-006-0106-0
Galluba S, Oehlmann J (2012) Widespread endocrine activity in river sediments in Hesse, Germany, assessed by a combination of in vitro and in vivo bioassays. J Soils Sediments 12(2):225–306. https://doi.org/10.1007/s11368-011-0465-x
Giudice BD, Young TM (2010) The antimicrobial triclocarban stimulates embryo production in the freshwater mudsnail Potamopyrgus antipodarum. Environ Toxicol Chem 29(4):966–970. https://doi.org/10.1002/etc.105
Gust M, Gagné F, Berlioz-Barbier A, Besse JP, Buronfosse T, Tournier M, Tutundjian R, Garric J, Cren-Olivé C (2014) Caged mudsnail Potamopyrgus antipodarum (Gray) as an integrated field biomonitoring tool: exposure assessment and reprotoxic effects of water column contamination. Water Res 54:222–236. https://doi.org/10.1016/j.watres.2014.01.057
Gust M, Buronfosse T, Geffard O, Mons R, Queau H, Mouthon J, Garric J (2010) In situ biomonitoring of freshwater quality using the New Zealand mudsnail Potamopyrgus antipodarum (Gray) exposed to waste water treatment plant (WWTP) effluent discharges. Water Res 44(15):4517–4528. https://doi.org/10.1016/j.watres.2010.06.019
Tuikka AI, Schmitt C, Höss S, Bandow N, Von der Ohe PC, De Zwart D, De Deckere E, Streck G, Mothes S, Van Hattum B, Kocan A, Brix R, Brack W, Barceló D, Sormunen AJ, Kukkonen JVK (2011) Toxicity assessment of sediments from three European river basins using a sediment contact test battery. Ecotoxicol Environ Saf 74(1):123–131. https://doi.org/10.1016/j.ecoenv.2010.08.038
Jobling S, Casey D, Rodgers-Gray T, Oehlmann J, Schulte-Oehlmann U, Pawlowski S, Baunbeck T, Turner AP, Tyler CR (2004) Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat Toxicol 66(2):207–222. https://doi.org/10.1016/j.aquatox.2004.01.002
Brettschneider D, Misovic A, Schulte-Oehlmann U, Oetken M, Oehlmann J (2019) Detection of chemically induced ecotoxicological effects in rivers of the Nidda catchment (Hessen, Germany) and development of an ecotoxicological, Water Framework Directive-compliant assessment system. Environ Sci Eur 31(7):1–22. https://doi.org/10.1186/s12302-019-0190-4
Harth FUR, Arras C, Brettschneider DJ, Misovic A, Oehlmann J, Schulte-Oehlmann U, Oetken M (2018) Small but with big impact? Ecotoxicological effects of a municipal waste water effluent on a small creek. J Environ Sci Health A 53(13):1149–1160. https://doi.org/10.1080/10934529.2018.1530328
Peschke K, Geburzi J, Köhler H-R, Wurm K, Triebskorn R (2014) Invertebrates as indicators for chemical stress in sewage-influenced stream systems: toxic and endocrine effects in gammarids and reactions at the community level in two tributaries of Lake Constance, Schussen and Argen. Ecotoxicol Environ Saf 106:115–125. https://doi.org/10.1016/j.ecoenv.2014.04.011
Ladewig V, Jungmann D, Köhler H-R, Schirling M, Triebskorn R, Nagel R (2006) Population structure and dynamics of Gammarus fossarum (Amphipoda) upstream and downstream from effluents of sewage treatment plants. Arch Environ Contam Toxicol 50(3):370–383. https://doi.org/10.1007/s00244-005-7039-0
Schmidt J (2003) Wirkung von Umweltchemikalien auf Gammarus fossarum—Populationsexperimente und individuenbasiertes Reproduktionsmodell. Dissertation, Technische Universität, Dresden, Deutschland
Schneider I, Oehlmann J, Oetken M (2015) Impact of an estrogenic sewage treatment plant effluent on life-history traits of the freshwater amphipod Gammarus pulex. J Environ Sci Health A 50(3):272–281. https://doi.org/10.1080/10934529.2015.981114
Watts MM, Pascoe D, Carroll K (2002) Population responses of the freshwater amphipod Gammarus pulex (L.) to an environmental estrogen, 17α-ethinylestradiol. Environ Toxicol Chem 21(2):445–450. https://doi.org/10.1002/etc.5620210230
Dietrich S, Dammel S, Ploessl F, Bracher F, Laforsch C (2010) Effects of a pharmaceutical mixture at environmentally relevant concentrations on the amphipod Gammarus fossarum. Mar Freshw Res 61(2):196. https://doi.org/10.1071/MF09048
Dedourge-Geffard O, Palais F, Biagianti-Risbourg S, Geffard O, Geffard A (2009) Effects of metals on feeding rate and digestive enzymes in Gammarus fossarum: an in situ experiment. Chemosphere 77(11):1569–1576. https://doi.org/10.1016/j.chemosphere.2009.09.042
Besse J-P, Coquery M, Lopes C, Chaumot A, Budzinski H, Labadie P, Geffard O (2013) Caged Gammarus fossarum (Crustacea) as a robust tool for the characterization of bioavailable contamination levels in continental waters: towards the determination of threshold values. Water Res 47(2):650–660. https://doi.org/10.1016/j.watres.2012.10.024
Wigh A, Geffard O, Abbaci K, Francois A, Noury P, Bergé A, Vulliet E, Domenjoud B, Gonzalez-Ospina A, Bony S, Devaux A (2017) Gammarus fossarum as a sensitive tool to reveal residual toxicity of treated wastewater effluents. Sci Total Environ 584–585:1012–1021. https://doi.org/10.1016/j.scitotenv.2017.01.154
Coulaud R, Geffard O, Xuereb B, Lacaze E, Quéau H, Garric J, Charles S, Chaumot A (2011) In situ feeding assay with Gammarus fossarum (Crustacea): modelling the influence of confounding factors to improve water quality biomonitoring. Water Res 45(19):6417–6429. https://doi.org/10.1016/j.watres.2011.09.035
Lacaze E, Devaux A, Mons R, Bony S, Garric J, Geffard A, Geffard O (2011) DNA damage in caged Gammarus fossarum amphipods: a tool for freshwater genotoxicity assessment. Environ Pollut 159(6):1682–1691. https://doi.org/10.1016/j.envpol.2011.02.038
OECD (1984) Guideline for testing of chemicals—Daphnia sp, acute immobilisation test and reproduction test (guideline 202, adopted 4th April 1984). OECD, Paris
Giebner S, Ostermann S, Straskraba S, Oetken M, Oehlmann J, Wagner M (2018) Effectivity of advanced wastewater treatment: reduction of in vitro endocrine activity and mutagenicity but not of in vivo reproductive toxicity. Environ Sci Pollut Res 25:3965–3976. https://doi.org/10.1007/s11356-016-7540-1
Abbas A, Schneider I, Bollmann A, Funke J, Oehlmann J, Prasse C, Schulte-Oehlmann U, Seitz W, Ternes T, Weber M, Wesely H, Wagner M (2019) What you extract is what you see: optimising the preparation of water and wastewater samples for in vitro bioassays. Water Res 152:47–60. https://doi.org/10.1016/j.watres.2018.12.049
Stalter D, Magdeburg A, Wagner M, Oehlmann J (2011) Ozonation and activated carbon treatment of sewage effluents: removal of endocrine activity and cytotoxicity. Water Res 45(3):1015–1024. https://doi.org/10.1016/j.watres.2010.10.008
Völker J, Vogt T, Castronovo S, Wick A, Ternes TA, Joss A, Oehlmann J, Wagner M (2017) Extended anaerobic conditions in the biological wastewater treatment: higher reduction of toxicity compared to target organic micropollutants. Water Res 116:220–230. https://doi.org/10.1016/j.watres.2017.03.030
DIN EN ISO 14688-1:2003-01, Geotechnische Erkundung und Untersuchung-Benennung, Beschreibung und Klassifizierung von Boden-Teil 1: Benennung und Beschreibung (ISO 14688-1:2002); Deutsche Fassung EN ISO 14688-1:2002. Beuth, Berlin, Deutschland
DIN 38414-3:1985-11, Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Sedimentuntersuchung-Schlamm und Sediment (Gruppe S)-Bestimmung des Glührückstandes und des Glühverlustes der Trockenmasse eines Schlammes (S 3). Beuth, Berlin, Deutschland
Stachel B, Jantzen E, Knoth W, Krüger F, Lepom P, Oetken M, Reincke H, Sawal G, Schwartz R, Uhlig S (2005) The Elbe flood in August 2002—organic contaminants in sediment samples taken after the flood event. J Environ Sci Health A 40(2):265–287. https://doi.org/10.1081/ESE-200045531
De Haas EM, Reuvers B, Moermond CTA, Koelmans AA, Kraak MHS (2002) Responses of benthic invertebrates to combined toxicant and food input in floodplain lake sediments. Environ Toxicol Chem 21(10):2165–2171. https://doi.org/10.1002/etc.5620211020
Stuijfzand SC, Helms M, Kraak MH, Admiraal W (2000) Interacting effects of toxicants and organic matter on the midge Chironomus riparius in polluted river water. Ecotoxicol Environ Saf 46(3):351–356. https://doi.org/10.1006/eesa.2000.1918
Besser JM, Brumbaugh WG, May TW, Ingersoll CG (2003) Effects of organic amendments on the toxicity and bioavailability of cadmium and copper in spiked formulated sediments. Environ Toxicol Chem 22(3):805–815. https://doi.org/10.1002/etc.5620220419
De Haas EM, Paumen ML, Koelmans AA, Kraak MHS (2004) Combined effects of copper and food on the midge Chironomus riparius in whole-sediment bioassays. Environ Pollut 127(1):99–107. https://doi.org/10.1016/S0269-7491(03)00252-5
Oetken M, Stachel B, Pfenninger M, Oehlmann J (2005) Impact of a flood disaster on sediment toxicity in a major river system—the Elbe flood 2002 as a case study. Environ Pollut 134(1):87–95. https://doi.org/10.1016/j.envpol.2004.08.001
Maltby L, Boxall ABA, Forrow DM, Calow P, Betton CI (1995) The effects of motorway runoff on freshwater ecosystems: 2. Identifying major toxicants. Environ Toxicol Chem 14(6):1093–1101. https://doi.org/10.1002/etc.5620140621
Fan W, Wang W-X, Chen J, Li X, Yen Y-F (2002) Cu, Ni, and Pb speciation in surface sediments from a contaminated bay of northern China. Mar Pollut Bull 44:816–832. https://doi.org/10.1016/S0025-326X(02)00069-3
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266:195–202. https://doi.org/10.1016/S0048-9697(00)00740-3
Kukkonen J, Oikari A (1991) Bioavailability of organic pollutants in boreal waters with varying levels of dissolved organic material. Water Res 25(4):455–463. https://doi.org/10.1016/0043-1354(91)90082-2
Yang W, Spurlock F, Liu W, Gan J (2006) Effects of dissolved organic matter on permethrin bioavailability to Daphnia species. J Agric Food Chem 54(11):3967–3972. https://doi.org/10.1021/jf060217y
Day KE (1991) Effects of dissolved organic carbon on accumulation and acute toxicity of fenvalerate, deltamethrin and cyhalothrin to Daphnia magna (Straus). Environ Toxicol Chem 10:91–101. https://doi.org/10.1002/etc.5620100111
Schmitt C, Balaam J, Leonards P, Brix R, Streck G, Tuikka A, Bervoets L, Brack W, Van Hattum B, Meire P, De Deckere E (2010) Characterizing field sediments from three European river basins with special emphasis on endocrine effects—a recommendation for Potamopyrgus antipodarum as test organism. Chemosphere 80(1):13–19. https://doi.org/10.1016/j.chemosphere.2010.03.050
Oehlmann J, Schulte-Oehlmann U, Bachmann J, Oetken M, Lutz I, Kloas W, Ternes TA (2006) Bisphenol A induces superfeminization in the ramshorn snail Marisa cornuarietis (Gastropoda: Prosobranchia) at environmentally relevant concentrations. Environ Health Perspect 114(suppl 1):127–133. https://doi.org/10.1289/ehp.8065
Schmitt C, Vogt C, Machala M, De Deckere E (2011) Sediment contact test with Potamopyrgus antipodarum in effect-directed analyses—challenges and opportunities. Environ Sci Pollut Res 18(8):1398–1404. https://doi.org/10.1007/s11356-011-0497-1
Maltby L, Forrow DM, Boxall ABA (1995) The effects of motorway runoff on freshwater ecosystems: 1. Field study. Environ Toxicol Chem 14(6):1079–1092. https://doi.org/10.1002/etc.5620140620
Boxall ABA, Maltby L (1997) The effects of motorway runoff on freshwater ecosystems: 3. Toxicant confirmation. Arch Environ Contam Toxicol 33:9–16. https://doi.org/10.1007/s002449900216
Hecker M, Giesy JP (2011) Effect-directed analysis of Ah-receptor mediated toxicants, mutagens, and endocrine disruptors in sediments and biota. In: Brack W (ed) Effect-directed analysis of complex environmental contamination, vol. 15, pp 285–314. https://doi.org/10.1007/978-3-642-18384-3_12
Alnafisi A, Hughes J, Wang G, Miller CA (2007) Evaluating polycyclic aromatic hydrocarbons using yeast bioassays. Environ Toxicol Chem 26(7):1333–1339. https://doi.org/10.1897/06-433R.1
Butler RA, Kelly ML, Powell WH, Hahn ME, Van Beneden RJ (2001) An aryl hydrocarbon receptor (AHR) homologue from the soft-shell clam, Mya arenaria: evidence that invertebrate AHR homologues lack 2,3,7,8-tetrachlordibenzo-p-dioxin and β-naphthoflavone binding. Gene 278:223–234. https://doi.org/10.1016/S0378-1119(01)00724-7
Miller RA, Norris LA, Hawkes CL (1973) Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in aquatic organisms. Environ Health Perspect 5:177–186. https://doi.org/10.2307/3428126
Cooper KR, Wintermyer M (2009) A critical review: 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) effects on gonad development in bivalve mollusks. J Environ Sci Health C 27(4):226–245. https://doi.org/10.1080/10590500903310112
Wu WZ, Li W, Xu Y, Wang JW (2001) Long-term toxic impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the reproduction, sexual differentiation, and development of different life stages of Gobiocypris rarus and Daphnia magna. Ecotoxicol Environ Saf 48(3):293–300. https://doi.org/10.1006/eesa.2000.2013
Ashley CM, Simpson MG, Holdich DM, Bell DR (1996) 2,3,7,8-Tetrachlor-dibenzo-p-dioxin is a potent toxin and induces cytochrome P450 in the crayfish, Pacifastacus leniusculus. Aquat Toxicol 35:157–169
Allinson G, Ueoka M, Morita M (1994) Effect of dietary 1,3,6,8-tetrachlordibenzo-p-dioxin on the japanese freshwater fish Oryzias latipex (Medaka) and aquatic snail Indoplanorbis exustus (Indohiramakigai). Chemosphere 28(7):1369–1383. https://doi.org/10.1016/0045-6535(94)90079-5
Mazurová E, Hilscherová K, Jálová V, Köhler H-R, Triebskorn R, Giesy JP, Bláha L (2008) Endocrine effects of contaminated sediments on the freshwater snail Potamopyrgus antipodarum in vivo and in the cell bioassays in vitro. Aquat Toxicol 89(3):172–179. https://doi.org/10.1016/j.aquatox.2008.06.013
Delgado-Moreno L, Wu L, Gan J (2015) Application of isotope dilution method for measuring bioavailability of organic contaminants sorbed to dissolved organic matter (DOM). Aquat Toxicol 165:129–135. https://doi.org/10.1016/j.aquatox.2015.05.006
Duft M, Schulte-Oehlmann U, Weltje L, Tillmann M, Oehlmann J (2003) Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. Aquat Toxicol 64(4):437–449. https://doi.org/10.1016/S0166-445X(03)00102-4
Lai KM, Johnson KL, Scrimshaw MD, Lester JN (2000) Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environ Sci Technol 34(18):3890–3894. https://doi.org/10.1021/es9912729
Duong CN, Schlenk D, Chang NI, Kim SD (2009) The effect of particle size on the bioavailability of estrogenic chemicals from sediments. Chemosphere 76(3):395–401. https://doi.org/10.1016/j.chemosphere.2009.03.024
Shi J, Liu X, Chen Q, Zhang H (2014) Spatial and seasonal distributions of estrogens and bisphenol A in the Yangtze River Estuary and the adjacent East China Sea. Chemosphere 111:336–343. https://doi.org/10.1016/j.chemosphere.2014.04.046
Nusair SD, Zarour YS, Altarifi AA (2017) Effects of dibenzo-p-dioxins/dibenzofurans on acetylcholinesterase activity and histopathology of the body wall of earthworm Eisenia andrei: a potential biomarker for ecotoxicity monitoring. Water Air Soil Pollut 228(7):1–11. https://doi.org/10.1007/s11270-017-3448-8
Sieratowicz A, Stange D, Schulte-Oehlmann U, Oehlmann J (2011) Reproductive toxicity of bisphenol A and cadmium in Potamopyrgus antipodarum and modulation of bisphenol A effects by different test temperature. Environ Pollut 159(10):2766–2774. https://doi.org/10.1016/j.envpol.2011.05.012
Dybdahl MF, Kane SL (2005) Adaption vs. phenotypic plasticity in the success of a clonal invader. Ecology 86(6):1592–1601. https://doi.org/10.1890/04-0898
Pöckl M (1993) Reproductive potential and lifetime potential fecundity of the freshwater amphipods Gammarus fossarum and G. roeseli in Austrian streams and rivers. Freshw Biol 30(1):73–91. https://doi.org/10.1111/j.1365-2427.1993.tb00790.x
Pöckl M, Humpesch UH (1990) Intra- and inter-specific variations in egg survival and brood development time for Austrian populations of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda). Freshw Biol 23(3):441–455. https://doi.org/10.1111/j.1365-2427.1990.tb00286.x
Holbrook RD, Love NG, Novak JT (2004) Sorption of 17β-estradiol and 17α-ethinylestradiol by colloidal organic carbon derived from biological wastewater treatment systems. Environ Sci Technol 38(12):3322–3329. https://doi.org/10.1021/es035122g
Laurenson JP, Bloom RA, Page S, Sadrieh N (2014) Ethinyl estradiol and other human pharmaceutical estrogens in the aquatic environment: a review of recent risk assessment data. AAPS J 16(2):299–310. https://doi.org/10.1208/s12248-014-9561-3
Völker J, Castronovo S, Wick A, Ternes TA, Joss A, Oehlmann J, Wagner M (2016) Advancing biological wastewater treatment: extended anaerobic conditions enhance the removal of endocrine and dioxin-like activities. Environ Sci Technol 50(19):10606–10615. https://doi.org/10.1021/acs.est.5b05732
Pardos M, Benninghoff C, De Alencastro LF, Wildi W (2004) The impact of a sewage treatment plant’s effluent on sediment quality in a small bay in Lake Geneva (Switzerland–France). Part 1: spatial distribution of contaminants and the potential for biological impacts. Lakes Reserv Res Manag 9:41–52. https://doi.org/10.1111/j.1440-1770.2004.00233.x
Young J, Iwanowicz L, Sperry A, Blazer V (2014) A landscape-based reconnaissance survey of estrogenic activity in streams of the upper Potomac, upper James, and Shenandoah Rivers, USA. Environ Monit Assess 186(9):5531–5545. https://doi.org/10.1007/s10661-014-3801-y
Shore LS, Shemesh M (2003) Naturally produced steroid hormones and their release into the environment. Pure Appl Chem 7511–12:1859–1871. https://doi.org/10.1351/pac200375111859
Eljarrat E, Caixach J, Rivera J (1997) Effects of sewage sludges contaminated with polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls on agricultural soils. Environ Sci Technol 31:2765–2771. https://doi.org/10.1021/es9610601
Rand GM, Carriger JF, Lee TA, Pfeuffer RJ (2004) Sediment toxicity in the St. Lucie river watershed and everglades agricultural area. Ecotoxicology 13:261–274. https://doi.org/10.1023/B:ECTX.0000023570.10555.49
van Emmerik T, Angove MJ, Johnson BB, Wells JD, Fernandes MB (2003) Sorption of 17β-estradiol onto selected soil minerals. J Colloid Interface Sci 266(1):33–39. https://doi.org/10.1016/S0021-9797(03)00597-6
Peck M, Gibson RW, Kortenkamp A, Hill EM (2004) Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environ Toxicol Chem 23(4):945–952
Keiter S, Rastall A, Kosmehl T, Erdinger L, Braunbeck T, Hollert H (2006) Ecotoxicological assessment of sediment, suspended matter and water samples in the Upper Danube River. A pilot study in search for the causes for the decline of fish catches (12 pp). Environ Sci Pollut Res 13(5):308–319. https://doi.org/10.1065/espr2006.04.300
Karlsson J, Sundberg H, Åkerman G, Grunder K, Eklund B, Breitholtz M (2008) Hazard identification of contaminated sites—ranking potential toxicity of organic sediment extracts in crustacean and fish. J Soils Sediments 8(4):263–274. https://doi.org/10.1007/s11368-008-0015-3
Chen G, White PA (2004) The mutagenic hazards of aquatic sediments: a review. Mutat Res 5672–3:151–225. https://doi.org/10.1016/j.mrrev.2004.08.005
Bunge M, Kähkönen MA, Rämisch W, Opel M, Vogler S, Walkow F, Salkinoja-Salonen M, Lechner U (2007) Biological activity in a heavily organohalogen-contaminated river sediment. Environ Sci Pollut Res 14:3–10
Apitz SE (2008) Adaptive management principles and sediment management. J Soils Sediments 8(6):359–362. https://doi.org/10.1007/s11368-008-0040-2
Juez C, Thalmann M, Schleiss AJ, Franca MJ, Paquier A, Rivière N (2018) Influence of lateral embayments on suspended sediment transport under unsteady flow conditions. In: E3S Web Conf, vol. 40, pp 3017. https://doi.org/10.1051/e3sconf/20184003017
Walling DE, Owens PN, Carter J, Leeks GJL, Lewis S, Meharg AA, Wright J (2003) Storage of sediment-associated nutrients and contaminants in river channel and floodplain systems. Appl Geochem 18:195–550. https://doi.org/10.1016/S0883-2927(02)00121-X
Maaß A-L, Esser V, Frings RM, Lehmkuhl F, Schüttrumpf H (2018) A decade of fluvial morphodynamics: relocation and restoration of the Inde River (North-Rhine Westphalia, Germany). Environ Sci Eur 30(1):40. https://doi.org/10.1186/s12302-018-0170-0
Extence CA, Chadd RP, England J, Dunbar MJ, Wood PJ, Taylor ED (2013) The assessment of fine sediment accumulation in rivers using macro-invertebrate community response. River Res Appl 29(1):17–55. https://doi.org/10.1002/rra.1569
Collins AL, Walling DE (2007) The storage and provenance of fine sediment on the channel bed of two contrasting lowland permeable catchments, UK. River Res Appl 23(4):429–450. https://doi.org/10.1002/rra.992
Gellis AC, Walling DE (2011) Sediment source fingerprinting (tracing) and sediment budgets as tools in targeting river and watershed restoration programs. Geophys Monogr Ser 194:263–291. https://doi.org/10.1029/2010GM000960
Denic M, Geist J (2015) Linking stream sediment deposition and aquatic habitat quality in pearl mussel streams: implications for conservation. River Res Appl 31(8):943–952. https://doi.org/10.1002/rra.2794
Collins AL, Walling DE, Leeks GJL (2005) Storage of fine-grained sediment and associated contaminants within channels of lowland permeable catchments in the UK. In: Sediment budgets 1. IAHS Publ, vol. 291. IAHS Press, Wallingford, pp 259–268
McMillan SK, Noe GB (2017) Increasing floodplain connectivity through urban stream restoration increases nutrient and sediment retention. Ecol Eng 108:284–295. https://doi.org/10.1016/j.ecoleng.2017.08.006
Wood PJ, Armitage PD (1997) Biological effects of fine sediment in the lotic environment. Environ Manag 21(2):203–217. https://doi.org/10.1007/s002679900019
Cheng P, Zhu H, Zhong B, Wang D (2015) Transport mechanisms of contaminants released from fine sediment in rivers. Acta Mech Sin 31(6):791–798. https://doi.org/10.1007/s10409-015-0520-8
Bundschuh M, Schletz M, Goedkoop W (2016) The mode of bioturbation triggers pesticide remobilization from aquatic sediments. Ecotoxicol Environ Saf 130:171–176. https://doi.org/10.1016/j.ecoenv.2016.04.013
Josefsson S, Leonardsson K, Gunnarsson JS, Wiberg K (2010) Bioturbation-driven release of buried PCBs and PBDEs from different depths in contaminated sediments. Environ Sci Technol 44(19):7456–7464. https://doi.org/10.1021/es100615g
Remaili TM, Simpson SL, Amato ED, Spadaro DA, Jarolimek CV, Jolley DF (2016) The impact of sediment bioturbation by secondary organisms on metal bioavailability, bioaccumulation and toxicity to target organisms in benthic bioassays: implications for sediment quality assessment. Environ Pollut 208(Pt B):590–599. https://doi.org/10.1016/j.envpol.2015.10.033
Hedman JE, Stempa Tocca J, Gunnarsson JS (2009) Remobilization of polychlorinated biphenyl from baltic sea sediment: comparing the roles of bioturbation and physical resuspension. Environ Toxicol Chem 28(11):2241–2249. https://doi.org/10.1897/08-576.1
Michaels RA, Oko UM (2017) Excessive PCBs in the Hudson river: attributable to incompleteness of dredging, or to seven years of dredging? Environ Claim J 29(2):115–140. https://doi.org/10.1080/10406026.2017.1307007
Van den Berg G, Meijers GGA, Van der Heijdt LM, Zwolsman JJG (2001) Dredging-related mobilisation of trace metals: a case study in the Netherlands. Water Res 35(8):1979–1986. https://doi.org/10.1016/S0043-1354(00)00452-8
Wölz J, Engwall M, Maletz S, Olsman Takner H, van Bavel B, Kammann U, Klempt M, Weber R, Braunbeck T, Hollert H (2008) Changes in toxicity and Ah receptor agonist activity of suspended particulate matter during flood events at the rivers Neckar and Rhine—a mass balance approach using in vitro methods and chemical analysis. Environ Sci Pollut Res 15(7):536–553. https://doi.org/10.1007/s11356-008-0056-6
Escher BI, Aїt-Aїssa S, Behnisch PA, Brack W, Brion F, Brouwer A, Buchinger S, Crawford SE, Du Pasquier D, Hamers T, Hettwer K, Hilscherová K, Hollert H, Kase R, Kienle C, Tindall AJ, Tuerk J, van der Oost R, Vermeirssen E, Neale PA (2018) Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci Total Environ 628–629:748–765. https://doi.org/10.1016/j.scitotenv.2018.01.340
Könemann S, Kase R, Simon E, Swart K, Buchinger S, Schlüsener M, Hollert H, Escher BI, Werner I, Aït-Aïssa S, Vermeirssen E, Dulio V, Valsecchi S, Polesello S, Behnisch P, Javurkova B, Perceval O, Di Paolo C, Olbrich D, Sychrova E, Schlichting R, Leborgne L, Clara M, Scheffknecht C, Marneffe Y, Chalon C, Tušil P, Soldàn P, von Danwitz B, Schwaiger J, San Martín Becares MI, Bersani F, Hilscherová K, Reifferscheid G, Ternes T, Carere M (2018) Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC 102:225–235. https://doi.org/10.1016/j.trac.2018.02.008
Kase R, Javurkova B, Simon E, Swart K, Buchinger S, Könemann S, Escher BI, Carere M, Dulio V, Ait-Aissa S, Hollert H, Valsecchi S, Polesello S, Behnisch P, Di Paolo C, Olbrich D, Sychrova E, Gundlach M, Schlichting R, Leborgne L, Clara M, Scheffknecht C, Marneffe Y, Chalon C, Tusil P, Soldan P, von Danwitz B, Schwaiger J, Palao AM, Bersani F, Perceval O, Kienle C, Vermeirssen E, Hilscherova K, Reifferscheid G, Werner I (2018) Screening and risk management solutions for steroidal estrogens in surface and wastewater. TrAC 102:343–358. https://doi.org/10.1016/j.trac.2018.02.013
Brack W, Escher BI, Müller E, Schmitt-Jansen M, Schulze T, Slobodnik J, Hollert H (2018) Towards a holistic and solution-oriented monitoring of chemical status of European water bodies: how to support the EU strategy for a non-toxic environment? Environ Sci Eur 30(1):33. https://doi.org/10.1186/s12302-018-0161-1
Carvalho RN, Arukwe A, Ait-Aissa S, Bado-Nilles A, Balzamo S, Baun A, Belkin S, Blaha L, Brion F, Conti D, Creusot N, Essig Y, Ferrero VEV, Flander-Putrle V, Fürhacker M, Grillari-Voglauer R, Hogstrand C, Jonáš A, Kharlyngdoh JB, Loos R, Lundebye A-K, Modig C, Olsson P-E, Pillai S, Polak N, Potalivo M, Sanchez W, Schifferli A, Schirmer K, Sforzini S, Stürzenbaum SR, Søfteland L, Turk V, Viarengo A, Werner I, Yagur-Kroll S, Zounková R, Lettieri T (2014) Mixtures of chemical pollutants at European legislation safety concentrations: how safe are they? Toxicol Sci 141(1):218–233. https://doi.org/10.1093/toxsci/kfu118
Välitalo P, Perkola N, Seiler T-B, Sillanpää M, Kuckelkorn J, Mikola A, Hollert H, Schultz E (2016) Estrogenic activity in Finnish municipal wastewater effluents. Water Res 88:740–749. https://doi.org/10.1016/j.watres.2015.10.056
Maletz S, Floehr T, Beier S, Klümper C, Brouwer A, Behnisch P, Higley E, Giesy JP, Hecker M, Gebhardt W, Linnemann V, Pinnekamp J, Hollert H (2013) In vitro characterization of the effectiveness of enhanced sewage treatment processes to eliminate endocrine activity of hospital effluents. Water Res 47(4):1545–1557. https://doi.org/10.1016/j.watres.2012.12.008
Brack W, Dulio V, Ågerstrand M, Allan I, Altenburger R, Brinkmann M, Bunke D, Burgess RM, Cousins I, Escher BI, Hernández FJ, Hewitt LM, Hilscherová K, Hollender J, Hollert H, Kase R, Klauer B, Lindim C, Herráez DL, Miège C, Munthe J, O’Toole S, Posthuma L, Rüdel H, Schäfer RB, Sengl M, Smedes F, van de Meent D, van den Brink PJ, van Gils J, van Wezel AP, Vethaak AD, Vermeirssen E, von der Ohe PC, Vrana B (2017) Towards the review of the European Union Water Framework management of chemical contamination in European surface water resources. Sci Total Environ 576:720–737. https://doi.org/10.1016/j.scitotenv.2016.10.104
Acknowledgements
The authors thank the NiddaMan collaboration partners, the fishing clubs of the Nidda catchment and the entire Department Aquatic Ecotoxicology at Goethe University for supporting biomonitoring campaigns, especially Simone Ziebart and Maren Lück.
Funding
This study was funded by the German Federal Ministry of Education and Research (BMBF) within the framework of the funding measures “Regional Water Resources Management for Sustainable Protection of Waters in Germany—ReWaM” and “Investigations of sustainable development—FONA” under the project “NiddaMan” (project identifier: 02WRM1367A).
Author information
Authors and Affiliations
Contributions
DB conducted active biomonitoring campaigns and laboratory experiments, prepared water and sediment samples, analyzed, interpreted and evaluated the gained in vivo and in vitro data and drafted the manuscript. AM prepared the extracts, conducted the in vitro screens and analyzed the data. MO supported the sampling campaigns and contributed to the interpretation and evaluation of the data. USO and JO supervised the project and the study, contributed to the interpretation and evaluation of the results and supported and improved the composing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As only invertebrates were used in the experiments of the present study, the German animal welfare act does not apply. Nevertheless, gammarids and mudsnails were handled with the utmost care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Figure S1.
Potamopyrgus antipodarum. Mean and standard deviation of the number of embryos (a, b) and mean and standard error of the mean of the percentage mortality (c, d) after 28 days of exposure in laboratory experiments with combined water/sediment samples of the river Horloff in March 2017 (a, c) and of the river Nidda in May 2017 (b, d). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in the number of embryos compared to the corresponding reference site H3R or N2R (shaded) were determined via Mann–Whitney test. *p < 0.05, n = 52.
Additional file 2: Figure S2.
Gammarus fossarum. Mean and standard deviation of the fecundity index (a, b) and mean and standard error of the mean of the percentage mortality (c, d) after 28 days of exposure in laboratory experiments with combined water/sediment samples of the river Horloff in March 2017 (a, c) and of the river Nidda in May 2017 (b, d). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in the percentage mortality in comparison to the corresponding reference site H3R or N2R (shaded) were detected using Fisher’s exact test. No mortality of gammarids occurred at H4 which is illustrated with “no bar”. *p < 0.05, **p < 0.01, ***p < 0.001, n = 4.
Additional file 3: Figure S3.
Mean and standard error of the mean of anti-estrogenic activities in water samples from the river Horloff in March 2017 (a) and the river Nidda in May 2017 (b). White: reference sites, with H3R and N2R as statistical reference sites, light grey: restoration sites, dark grey: transect sites. Significant differences in anti-estrogenic activity compared to the reference site H3R (shaded) were determined using Kruskal–Wallis test with Dunn’s post hoc test. Significant differences in anti-estrogenic activity compared to the reference site N2R (shaded) were detected via one-way ANOVA and Bonferroni’s post hoc test. If no bar is illustrated, the activity was below the corresponding LOQ. *p < 0.05, **p < 0.01, ***p < 0.001, n = 3 with 8 pseudo-replicates each.
Additional file 4: Table S1.
Associated water parameters in the active biomonitoring campaigns at the river Horloff in March 2017 and the river Nidda in May 2017. H1-H3R and N1-N2R: reference sites, with H3R and N2R as statistical reference sites, H4 and N3-N5: restoration sites, H5-H9 and N6-N9: transect sites. Table S2. Average associated water parameters in laboratory experiments with Gammarus fossarum and combined water/sediment samples from the river Horloff in March 2017 and the river Nidda in May 2017. H1-H3R and N1-N2R: reference sites, with H3R and N2R as statistical reference sites, H4 and N3-N5: restoration sites, H5-H9 and N6-N9: transect sites. Table S3. Average associated water parameters in laboratory experiments with Potamopyrgus antipodarum and combined water/sediment samples from the river Horloff in March 2017 and the river Nidda in May 2017. H1-H3R and N1-N2R: reference sites, with H3R and N2R as statistical reference sites, H4 and N3-N5: restoration sites, H5-H9 and N6-N9: transect sites. Table S4. Limits of quantification (LOQ) for in-vitro assays with water or sediment samples.
Additional file 5: Figure S4.
Linear correlation analysis between explanatory variables (estrogenic and dioxin-like activity in water samples, dioxin-like activity in sediment samples, mean grain size, loss on ignition) and the response variables (number of embryos of Potamopyrgus antipodarum and fecundity index of Gammarus fossarum).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brettschneider, D.J., Misovic, A., Schulte-Oehlmann, U. et al. Poison in paradise: increase of toxic effects in restored sections of two rivers jeopardizes the success of hydromorphological restoration measures. Environ Sci Eur 31, 36 (2019). https://doi.org/10.1186/s12302-019-0218-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-019-0218-9