Abstract
Background
The chemical control of the mosquito Aedes aegypti, the major vector of dengue, is being seriously threatened due to the development of pyrethroid resistance. Substitutions in the 1016 and 1534 sites of the voltage gated sodium channel (AaNaV), commonly known as kdr mutations, confer the mosquito with knockdown resistance. Our aim was to evaluate the allelic composition of natural populations of Brazilian Ae. aegypti at both kdr sites.
Methods
The AaNaV IIIS6 region was cloned and sequenced from three Brazilian populations. Additionally, individual mosquitoes from 30 populations throughout the country were genotyped for 1016 and 1534 sites, based in allele-specific PCR. For individual genotypes both sites were considered as a single locus.
Results
The 350 bp sequence spanning the IIIS6 region of the AaNa V gene revealed the occurrence of the kdr mutation Phe1534Cys in Brazil. Concerning the individual genotyping, beyond the susceptible wild-type (NaVS), two kdr alleles were identified: substitutions restricted to the 1534 position (NaVR1) or simultaneous substitutions in both 1016 and 1534 sites (NaVR2). A clear regional distribution pattern of these alleles was observed. The NaVR1kdr allele occurred in all localities, while NaVR2 was more frequent in the Central and Southeastern localities. Locations that were sampled multiple times in the course of a decade revealed an increase in frequency of the kdr mutations, mainly the double mutant allele NaVR2. Recent samples also indicate that NaVR2 is spreading towards the Northern region.
Conclusions
We have found that in addition to the previously reported Val1016Ile kdr mutation, the Phe1534Cys mutation also occurs in Brazil. Allelic composition at both sites was important to elucidate the actual distribution of kdr mutations throughout the country. Studies to determine gene flow and the fitness costs of these kdr alleles are underway and will be important to better understand the dynamics of Ae. aegypti pyrethroid resistance.
Similar content being viewed by others
Background
Dengue is currently the most important arbovirus in the world. Dengue has spread widely in urban areas of tropical and subtropical regions during the last decades, including countries of Southeast Asia, Pacific and Latin America [1]. Between 2001–2011, almost 10 million dengue cases were reported in Latin America, almost 60% of these were registered in Brazil [2]. Dengue mortality can reach up to 5% of the confirmed infection cases. In addition, in tropical dengue endemic countries a loss of 1,300 disability-adjust life years (DALYs) per million people is estimated [1]. Aedes aegypti is the main dengue vector throughout the world. Control of this mosquito consists primarily of the elimination of artificial and disposable water flooded larvae breeding sites and application of insecticides. The WHO Pesticide Evaluate Scheme (WHOPES) recommends ten different compounds to eliminate larvae, including neurotoxicants (organophosphates, pyrethroids and neocotinoids), Insect Growth Regulators (chitin synthesis inhibitors and juvenile hormone analogs), and Bacillus (like B. thuringiensis var israelensis) as larvicides. However, fewer formulations are recommended for the control of adult mosquitoes, mostly five pyrethroids and one organophosphate [3].
Given their rapid mode of action and low hazardous effect to the environment, compared to organophosphate insecticides, the use of pyrethroids has increased significantly in the last two decades. Nowadays, pyrethroids are widely employed in and around households, even for pet protection and mosquito control [4]. Since Ae. aegypti is essentially an urban mosquito, it is constantly exposed to strong pyrethroid selection. As a consequence, many Ae. aegypti populations worldwide are becoming resistant to this class of insecticides [5].
Pyrethroids target the transmembrane voltage gated sodium channel (NaV) from the insect nervous system, triggering rapid convulsions followed by death, a phenomenon known as knockdown effect [6]. The NaV is composed of four homologous domains (I-IV), each with six hydrophobic segments (S1-S6) [7]. Because the NaV is a very conserved protein among invertebrates, small changes are permissive without impairing its physiological role [8]. A series of mutations have been identified in different orders of insects and acarids that affect pyrethroid susceptibility, thus being referred to as ‘k nockd own r esistance’ or kdr mutations [9]. These kdr mutations may lead to conformational changes in the whole channel that maintain its physiological role but avoid insecticide action [10].
In insects, the most common kdr mutation is the substitution Leu/Phe in the 1014 site (numbered according to the Musca domestica NaV primary sequence), followed by the Leu/Ser substitution in the same position, in Anopheles and Culex mosquitoes [11]. In the Ae. aegypti NaV (AaNa V ), the 1014 Leu codon is encoded by CTA, rather than TTA as in Anopheles and Culex mosquitoes. This means that two substitutions would have to be simultaneously selected in the same codon in order to change Leu to Phe (TTT) or Ser (TCA) [12]. Although several mutations have been identified in natural populations at AaNa V [13], only the Val1016Ile and Phe1534Cys substitutions were clearly related to the loss of pyrethroid susceptibility [12, 14]. These sites are placed respectively in the IIS6 and IIIS6 regions of the channels that are known to be involved in the interaction with pyrethroids [10]. It has been previously observed that in Latin America the 1016 Ile kdr is highly disseminated [12, 15, 16] and its frequency is rapidly increasing in localities with intense pyrethroid use, such as Brazil and Mexico [15, 16]. High frequencies of 1534 Cys kdr were also observed in Grand Cayman and Martinique [14, 17].
In the current study, we demonstrate that the1534 Cys kdr mutation is present in Brazil together with the 1016 Ile allele previously found. The simultaneous occurrence of both kdr mutations at the 1016 and 1534 was found in several localities. Spatial and temporal analysis of these alleles point to a significant role of the kdr mutations in pyrethroid resistance in Brazil.
Methods
Mosquito samples
Ae. aegypti used for kdr genotyping originated from the same samples evaluated by the Brazilian Aedes agypti Insecticide Resistance Monitoring Network, collected with ovitraps according to recommendations of the Brazilian Dengue Control Program [18]. Adult mosquitoes resulting from the eggs collected in the field (F0 generation) were preferentially used. However, in some cases only the following generations reared in the laboratory were available. Details regarding sampling as well as individual data from mosquitoes used for kdr genotyping are found in Table 1. A total of 30 localities were analyzed at least once, with AJU, SGO, MSR and VIT analyzed for two-four time-points.
Genotyping assays
Thirty individual mosquitoes from each locality were genotyped at both 1016 and 1534 positions from genomic DNA by allele-specific PCR (AS-PCR) which contains a common primer and two specific primers targeting each polymorphic site. The specificity is attained in the 3′-end, strengthened by a transition three nucleotides before [19]. Additionally, a GC-tail of different sizes was added at the 5′-end of these primers so products can be distinguished by their melting temperature (Tm) in a melting curve analysis or by electrophoresis [12, 20, 21]. Primer sequences are shown in Table 2. DNA extraction and amplification of the 1016 (Val/Ile) site were conducted as previously described [15]. The reaction for the 1534 (Phe/Cys) site was optimized from previous work [16, 22]. In both cases, PCR was carried out with the GoTaq Green Master Mix kit (Promega), 0.5 μL of genomic DNA, 0.24 μM of the common primer, 0.12 and 0.24 μM of the specific primers (1534 Cyskdr and 1534 Phe), in a total volume of 12.5 μL. Denaturing, annealing and extension conditions were, respectively, 95°C ⁄ 30″, 54°C ⁄ 40″ and 72°C ⁄ 45″, in 32 cycles. Alternatively, real-time PCR was conducted with the SYBR Green PCR Master Mix kit (LifeTechnologies/Applied Biosystems), 1 μL genomic DNA and 0.24 μM of each primer, in a total volume of 10 μL. The best conditions for denaturing, annealing and extension were respectively 95°C ⁄ 15″, 54°C ⁄15″ and 60°C ⁄ 30″, in 33 cycles, followed by a standard melting curve stage. The amplification reaction and melting curve analyses were performed in a StepOne Plus or in a 7500 Real-time PCR system (LifeTechnologies/Applied Biosystems). DNA pools of individuals from CGR, STR and PNM were used to amplify the region spanning the NaV IIIS6 segment with the primers AaEx31P and AaEx31Q (Table 2), as specified elsewhere [14]. The PCR products were purified in S-400 microcolumns (GE Healthcare) according to manufacturer instructions and cloned with CloneJet PCR Cloning Kit (Thermo Scientific). The DNA sequencing was carried out in an ABI377 Sequencer with the Big Dye 3.1 Kit (LifeTechnologies/Applied Biosystems). Sequence analysis was performed using the BioEdit software version 7.2.
All individuals were genotyped for both 1016 and 1534 sites. Linkage disequilibrium was tested by the online Genepop version 4.2 [23], and since the 1016 and 1534 sites are linked (see Results section), genotypic and allelic frequencies were taken as a single locus. Hardy-Weinberg equilibrium was evaluated by the classical equation [24], being the null hypothesis of equilibrium checked by a chi-square test with three or one degrees of freedom, respectively, when six or three genotypes were evidenced.
Results
Allele-specific discrimination
A 20 bp size difference, due to the 5′-GC tail of allele specific primers, enabled the easy discrimination of homozygous and heterozygous genotypes in either a polyacrylamide gel electrophoresis or in dissociation curves through real-time PCR (Figure 1). Electrophoresis revealed products of around 80 and 100 bp, respectively for Ilekdr and Val+ (1016 reaction), and 90 and 110 bp, respectively for Phe+ and Cyskdr (1534 reaction). The dissociation curve exhibited Tm of around 76 and 84°C, respectively for Ilekdr and Val (1016 reaction), and 77 and 82°C, respectively for Phe and Cyskdr (1534 reaction). The PCR conditions of annealing temperature, number of cycles and concentration of each primer were crucial to avoid unspecific amplification. All reactions were accompanied by positive controls, each one consisting of the three potential genotypes at the 1016 and 1534 positions, which were obtained by previously genotyped individuals: homozygous wild type, heterozygous, and homozygous kdr. As the Phe1534Cys mutation was detected for first time in Brazilian samples, we cloned and sequenced the IIIS6 region (exon 31) of the AaNa V gene of three genotyped populations (CGR, STR and PNM), confirming the primers’ specificity. The 350 bp fragments were submitted to GenBank (accession numbers: KF527414 and KF527415, for 1534 Cyskdr and 1534 Phe+, respectively). Excluding the site of the 1534 kdr mutation (TTC/TGC), no other polymorphic site was detected relative to the sequence deposited in VectorBase (Liverpool strain).
Allele specific PCR (AS-PCR) for genotyping kdr mutations in the Aedes aegypti voltage gated sodium channel. All panels represent reactions for the 1534 site. (A) Visualization of the amplicons in a 10% polyacrylamide gel electrophoresis, run under 170 V/45' and stained with ethidium bromide (1 μg/mL). Amplicons of approximately 90 and 110 bp correspond to alleles 1534 Phe+ and 1534 Cyskdr, respectively. DNA ladder was used as size marker (O’GeneRuler DNA Ladder, Ultra Low Range/Fermentas, 150 ng). Dissociation curve analysis in real time PCR differentiating the Phe/Phe (B), Phe/Cys (C), and Cys/Cys (D) genotypes. The Tm for the respective alleles are indicated.
Genotyping 1016 and 1534 Aa NaV sites in natural populations
Around 30 Ae. aegypti individuals from each one of 30 distinct Brazilian localities were genotyped for both 1016 and 1534 NaV sites, totalling 1,112 analyzed mosquitoes. Some localities were sampled two to four times within a ten-year interval. The genotypes of individual mosquitoes for both sites were first calculated independently: 1016 Val+/Val+, Val+/Ilekdr and Ilekdr/Ilekdr, and 1534 Phe+/Phe+, Phe+/Cyskdr and Cyskdr/Cyskdr. These data were used to perform a genotypic linkage disequilibrium analysis and total linkage between them was demonstrated (Fisher’s method, p < 0.001), as expected from two sites placed in the same gene. In this sense both sites were considered as constituents of a single locus, thus evidencing the occurrence of six genotypes in individual mosquitoes (Table 3). Based on the composition of these genotypes, we concluded that three alleles were present in the evaluated samples: ‘1016 Val+ + 1534 Phe+’ (wild-type), ‘1016 Val+ + 1534 Cyskdr’ (1534 kdr) and ‘1016 Ilekdr + 1534 Cyskdr’ (1016 kdr + 1534 kdr). Hereafter these alleles will be simply referred to as ‘NaVS’, ‘NaVR1’ and ‘NaVR2’, respectively (Figure 2). Double mutants and individuals with mutation only in the 1534 position were found (respectively, NaVR2 and ‘NaVR1); however, in no case was the 1016 kdr mutation observed alone, precluding the existence of a 1016 Ilekdr + 1534 Phe+ allele in the evaluated populations. Figure 3 shows the frequencies for NaVS, NaVR1 and NaVR2 alleles in the most recent samples obtained from each locality. The 95% CI of the allele frequencies is shown in the Additional file 1: Table S1. According to the alleles, the genotypes were named SS, SR1, SR2, R1R1, R1R2 and R2R2. Their frequencies and the Hardy-Weinberg Equilibrium deviation test are presented in Table 3. In only seven out of 38 samplings the Hardy-Weinberg Equilibrium assumption was rejected (p < 0.05). No specific genotype contributed to the deviation in these seven localities.
Voltage gated sodium channel and the 1016 and 1534 alleles found in Brazilian Aedes aegypti populations. The NaV is represented with its four domains (I-IV), each with the six transmembrane segments (S1-S6). The voltage sensitive S4 and the pore forming S6 segments are colored in blue and green, respectively (scheme adapted from [9]). The 1016 and 1534 kdr sites in Aedes aegypti are indicated. Mutant amino acids are underlined.
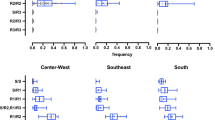
Overall, the distribution of the three alleles differed according to the geographical region (Figure 3). In the North and Northeast Regions, the NaVR1 allele, mutant only at position 1534, was found in all localities, nevertheless the NaVS wild-type allele was the most representative in six of the localities (BEL, CTL, MRB, CAC, SIP and PNM). The highest frequency of NaVR1, was found in the North: 0.750 (STR), among all populations analyzed. On the other hand, with exception of the most recent AJU (AJU2012), the NaVR2 double mutant allele was either absent or < 5% in the North and Northeast of Brazil. In contrast, the wild-type allele, NaVS, was absent from the two northernmost localities evaluated (PCR and BVT, both in the State of Roraima), where both mutant alleles were at high frequencies. In all localities from Central-West, Southeast and South regions, all three alleles were present. The most frequent allele was the NaVR2 double mutant. Exceptions were LZN, SMA, URU, SGO and SRO, where the NaVS wild-type allele was the most representative (Figure 3).
The dynamics of the genotype frequencies was analyzed in AJU, MSR, VIT and DQC. Samples from AJU were collected four times in the course of a decade, between 2002 and 2012. In 2002, only the NaVS wild-type allele was detected. The kdr alleles appeared first in 2006 and the double mutant NaVR2 was the most frequent allele by 2012 (Figure 4). Accordingly, the ‘SS’ wild-genotype progressively decayed from 100% in 2002 to 20% in 2012, when the double mutant ‘R2R2’ represented 30% of the individuals, and was the most frequent genotype (AJU2012, Table 3). The frequency of the NaVS wild-type allele also decreased in all other localities evaluated where the kdr alleles increased in frequency (Figure 4). Except for MSR, the NaVR2 double mutant is likely to be the most favorably selected allele. It is noteworthy that in AJU, the NaVR1 allele showed the larger frequency increase, probably because NaVR2 must have arrived to the Northeast more recently.
Discussion
The genotyping of mutations directly related to insecticide resistance is an important surveillance tool for agricultural and sanitary purposes. Among selected mechanisms of pyrethroid resistance, kdr mutations in the voltage gated sodium channel (NaV) are those that better correlate particular genotypes with insecticide resistance [25]. The increased efficiency of insecticide detoxification, known as metabolic resistance – involving super families of enzymes such as GST, esterases and especially the multi function oxidases P450 – may also confer resistance to pyrethroids. However, identification of these mechanisms is mainly based on enzymatic assays of low specificity [26] or on bioassays with synergist compounds [27], and are not clearly linked to particular genes. More recently, many successful transcriptome tools for metabolic resistance genes have emerged, pointing to a very complex and diverse scenario regarding insecticide selected genes and their pattern of expression among insect populations [28, 29]. Because the metabolic resistance based selection seems to have a high fitness cost, due to reallocation of energetic resources, this mechanism is expected to induce lower resistance levels, if compared to mutations in the target site molecules [30]. This was corroborated by laboratory selection with pyrethroids in an Ae. aegypti lineage: increase of the 1016 Ilekdr frequency was inversely proportional to the number of ‘metabolic’ genes differentially transcribed [29]. It was hypothesized that, in the presence of pyrethroid, kdr mutations are preferentially selected among other mechanisms, contributing to higher resistance levels and/or resulting in less deleterious effects.
In addition to the classical Leu1014Phe kdr mutation, several others have been associated with pyrethroid resistance [6]. Interactions of multiple NaV mutations may modulate pyrethroid resistance levels. For instance, certain NaV haplotypes, including synonymous substitutions, were found in two distinct field populations of Culex quinquefasciatus selected for pyrethroid resistance during 6–8 generations in the laboratory. It was suggested that some of these haplotypes were selected at an early stage of permethrin resistance and later evolved to other mutation combinations in the course of selection pressure [31]. In Ae. aegypti, a synonymous substitution at exon 20, together with an extensive polymorphism in the following intron, were linked to both Ile1011Met and Val1016Ile mutations [15, 32]. Additionally, a gene duplication event was recently described in the AaNa V of natural populations and in a laboratory strain selected for pyrethroid resistance [33]. Although there are at least seven different mutations described in the AaNa V , only those corresponding to the 1016 and 1534 positions are clearly related to resistance; both are placed in a domain of the sodium channel that interacts directly with the pyrethroid molecule [34].
There are two mutations described in the Aa NaV 1016 site, Val to Ile or Gly, respectively in Latin America [12, 14, 15] and in Southeast Asia [35]. In Brazil, we found no evidence of a haplotype that contains exclusively the 1016 Ilekdr mutation, since it was always found together with 1534 Cyskdr (NaVR2 allele, herein). Nevertheless, we are aware that it is possible for a haplotype carrying the 1016 kdr mutation to occur in the populations examined, however, it would be present at very low frequencies. Actually, this putative allele must have occurred in two out of three Ae. aegypti populations from Grand Cayman, given that the 1016 Ilekdr presented a higher frequency than the 1534 Cyskdr substitution [14].
Differently from the 1016 position, only one substitution, Phe/Cys, was found in the 1534 site by far [14, 36]. This 1534 substitution can be linked with another one. In Thailand, the 1534 Cyskdr co-occurred with 1016 Glykdr and 989 Prokdr in the same molecule [22]. In that region an allele 1534 Cyskdr without mutation in 1016 site (NaVR1 allele, herein) seemed to be very common, since its frequency was higher than the 1016 Glykdr[35].
Here we presented the distribution of the kdr variants for the AaNaV, considering both 1016 and 1534 sites screen from several natural Brazilian populations. We considered that once these sites are very close in the genome, reporting the allele/genotypic frequencies of each site separately would not be fully informative. However, because there are still some gaps concerning the actual role of these mutations in pyrethroid resistance, regarding whether they are acting alone or synergistically, and present in cis or trans mutations, we are reporting the allele frequencies of each site rather than as an haplotype. The implication of the 1016 Ilekdr allele in resistance to pyrethroids was corroborated by laboratory selection, which highly increased the allelic frequency up to fixation in only five generations [29]. Accordingly, in the last decade this mutation has been rapidly spreading in natural populations from Brazil and Mexico, concomitantly with the intensification of pyrethroid usage due to the emergence of severe dengue outbreaks [15, 16]. In these cases however, the co-occurrence of the 1534 Cyskdr mutation has been overlooked. A recent study reported high frequencies of 1534 Cyskdr in Grand Cayman [14], suggesting it is not a novel mutation in Latin America. In a recent report, nine single and two double AaNa V mutants were constructed and inserted in a Xenopus oocyte system in order to perform functional evaluations of these substitutions in the presence of type I or II pyrethroids [34]. The 1016 Ilekdr construct did not result in sensitivity reduction, to either pyrethroid types. On the other hand, the 1534 Cyskdr significantly diminished the AaNaV sensibility to type I but not to type II pyrethroids. This same substitution in the homologous kdr site of the cockroach NaV exhibited similar results [37].
An Ae. aegypti lineage, selected for permethrin resistance in the laboratory, exhibited high frequencies of 1016 Glykdr + 1794 Tyrkdr substitutions in the same molecule, which suggested a synergistic effect towards pyrethroid resistance [38]. We hypothesize that mutation in the 1016 site should be important when in synergism with other specific mutations. In Brazil, the 1534 Cyskdr mutation is widespread throughout the territory. The NaVR1 allele is more frequent in North/Northeast regions whereas NaVR2 is more commonly present in Central/Southeast regions, generally where the highest resistance levels to pyrethroids are observed [18]. Both mutant haplotypes appear to be rapid and favorably selected in all evaluated populations. However, in the most recent samplings the NaVR2 double mutant was the more frequent kdr allele. The exception was MSR, in the Northeast Region, where NaVR2 was only recently introduced. Together these data suggest that NaVR2 allele would be more advantageous for pyrethroid resistance, or impose a lower fitness cost when compared to NaVR1. We recently demonstrated that an NaVR2 homozygous Ae. aegypti lineage, highly resistant to pyrethroids, exhibited a fitness cost in a series of life-trait parameters [39]. Further comparisons between NaVR1 and NaVR2 lineages will be of importance to better clarify those assumptions.
It is of note that since 2001 and up to 2009 the Brazilian Dengue Control Program employed pyrethroids in ultralow volume applications in several municipalities as part of the effort to control the dengue vector [18]. With very few exceptions, the basis for pyrethroid selection pressure derived from national campaigns is essentially the same in the whole country. Therefore, differential selection pressures would not explain the aforementioned regionalization of the kdr alleles. It is likely that the current distribution of the kdr alleles reflects distinct Ae. aegypti populations that colonized the continent. Population genetics analysis of neutral loci will help us to unravel the evolutionary routes of these resistance genes.
Conclusions
In conclusion, pyrethroids are the most employed insecticides worldwide and the only chemical class presently allowed in long lasting treated materials, such as nets and curtains [40]. Although novel control strategies are being tested in the field, such as those based on transgenic and on Wolbachia-infected mosquitoes [2, 41, 42], insecticides will certainly play an important role for yet a long time. Knowledge of the sodium channel diversity in natural populations together with the role of each allele regarding pyrethroid resistance as well as their fitness effects are crucial for preserving the effectiveness of this class of compounds as a viable tool against Ae. aegypti.
References
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J: (2010) Dengue: a continuing global threat. Nat Rev Microbiol. 2010, 8: S7-S16. 10.1038/nrmicro2460.
Maciel-de-Freitas R, Aguiar R, Bruno RV, Guimaraes MC, Lourenco-de-Oliveira R: Why do we need alternative tools to control mosquito-borne diseases in Latin America?. Mem Inst Oswaldo Cruz. 2012, 107: 828-829. 10.1590/S0074-02762012000600021.
WHOPES: Pesticides and their application for the control of vectors and pests of public health importance (WHO/CDS/NTD/WHOPES/GCDPP/2006.1). 2006, Geneva: World Health Organization
Agency USEP: Pesticides: Regulating Pesticides. 2012, U.S. Environmental Protection Agency,http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html,
Ranson H, Burhani J, Lumjuan N, Black WC: Insecticide resistance in dengue vectors. TropIKAnet. 2010, 1: 1-cited 2013-12-02], pp. 0–0. Available from: http://journal.tropika.net/scielo.php?script=sci_arttext&pid=S2078-86062010000100003&lng=en&nrm=iso. ISSN 2078–8606
Dong K: Insect sodium channels and insecticide resistance. Invert Neurosci. 2007, 7: 17-30. 10.1007/s10158-006-0036-9.
Catterall WA: From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000, 26: 13-25. 10.1016/S0896-6273(00)81133-2.
Pittendrigh B, Vaughan A, Anthony N, ffrench-Constant RH: Why are there so few resistance-associated mutations in insecticide target genes?. Philos Trans R Soc Lond B Biol Sci. 1998, 353: 1685-1693. 10.1098/rstb.1998.0319.
Martins AJ, Valle D: The pyrethroid knockdown resistance. Insecticides - Basic and Other Applications. Edited by: Soloneski S, Larramendy M. 2012, Rijeka: InTech, 17-38.
O’Reilly AO, Khambay BP, Williamson MS, Field LM, Wallace BA: Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem J. 2006, 396: 255-263. 10.1042/BJ20051925.
Davies TG, Field LM, Usherwood PN, Williamson MS: A comparative study of voltage-gated sodium channels in the Insecta: implications for pyrethroid resistance in Anopheline and other Neopteran species. Insect Mol Biol. 2007, 16: 361-375. 10.1111/j.1365-2583.2007.00733.x.
Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE: A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007, 16: 785-798. 10.1111/j.1365-2583.2007.00774.x.
Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S: Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003, 17: 87-94. 10.1046/j.1365-2915.2003.00412.x.
Harris AF, Rajatileka S, Ranson H: Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg. 2010, 83: 277-284. 10.4269/ajtmh.2010.09-0623.
Martins AJ, Lima JB, Peixoto AA, Valle D: Frequency of Val1016Ile mutation in the voltage-gated sodium channel gene of Aedes aegypti Brazilian populations. Trop Med Int Health. 2009, 14: 1351-1355. 10.1111/j.1365-3156.2009.02378.x.
Garcia GP, Flores AE, Fernandez-Salas I, Saavedra-Rodriguez K, Reyes-Solis G: Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2009, 3: e531-10.1371/journal.pntd.0000531.
Marcombe S, Mathieu RB, Pocquet N, Riaz MA, Poupardin R: Insecticide resistance in the dengue vector Aedes aegypti from Martinique: distribution, mechanisms and relations with environmental factors. PLoS One. 2012, 7: e30989-10.1371/journal.pone.0030989.
Montella IR, Martins AJ, Viana-Medeiros PF, Lima JB, Braga IA: Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am J Trop Med Hyg. 2007, 77: 467-477.
Okimoto R, Dodgson JB: Improved PCR amplification of multiple specific alleles (PAMSA) using internally mismatched primers. Biotechniques. 1996, 21: 20-22. 24, 26
Germer S, Higuchi R: Single-tube genotyping without oligonucleotide probes. Genome Res. 1996, 9: 72-78.
Wang J, Chuang K, Ahluwalia M, Patel S, Umblas N: High-throughput SNP genotyping by single-tube PCR with Tm-shift primers. Biotechniques. 2005, 39: 885-893. 10.2144/000112028.
Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P: High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop Med Int Health. 2011, 16: 501-509. 10.1111/j.1365-3156.2011.02725.x.
Raymond M, Rousset F: Genepop (Version-1.2) - population-genetics software for exact tests and ecumenicism. J Hered. 1995, 86: 248-249.
Shorrocks B: The Genesis of Diversity. 1978, London: Hodder and Stoughton
Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS: Does kdr genotype predict insecticide-resistance phenotype in mosquitoes?. Trends Parasitol. 2009, 25: 213-219. 10.1016/j.pt.2009.02.007.
Valle D, Montella IR, Medeiros PFV, Ribeiro RA, Martins AJ: Quantification methodology for enzyme activity related to insecticide resistance in Aedes aegypti. 2006, Ministério da Saúde/Brasil: Brasília
Brogdon WG, McAllister JC: Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc. 1998, 14: 159-164.
Bariami V, Jones CM, Poupardin R, Vontas J, Ranson H: Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl Trop Dis. 2012, 6: e1692-10.1371/journal.pntd.0001692.
Saavedra-Rodriguez K, Suarez AF, Salas IF, Strode C, Ranson H: Transcription of detoxification genes after permethrin selection in the mosquito Aedes aegypti. Insect Mol Biol. 2012, 21: 61-77. 10.1111/j.1365-2583.2011.01113.x.
Martins AJ, Ribeiro CD, Bellinato DF, Peixoto AA, Valle D: Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS One. 2012, 7: e31889-10.1371/journal.pone.0031889.
Xu Q, Zhang L, Li T, Zhang L, He L: Evolutionary adaptation of the amino acid and codon usage of the mosquito sodium channel following insecticide selection in the field mosquitoes. PLoS One. 2012, 7: e47609-10.1371/journal.pone.0047609.
Martins AJ, Lins RM, Linss JG, Peixoto AA, Valle D: Voltage-gated sodium channel polymorphism and metabolic resistance in pyrethroid-resistant Aedes aegypti from Brazil. Am J Trop Med Hyg. 2009, 81: 108-115.
Martins AJ, Brito LP, Linss JGB, Rivas GB, Machado R: Evidence for gene duplication in the voltage gated sodium channel gene of Aedes aegypti. EMPH. 2013, eoto12v1-eot012.
Du Y, Nomura Y, Satar G, Hu Z, Nauen R: Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci USA. 2013, 1305118110v1-1305118110v201305118.
Kawada H, Higa Y, Komagata O, Kasai S, Tomita T: Widespread distribution of a newly found point mutation in voltage-gated sodium channel in pyrethroid-resistant Aedes aegypti populations in Vietnam. PLoS Negl Trop Dis. 2009, 3: e527-10.1371/journal.pntd.0000527.
Yanola J, Somboon P, Walton C, Nachaiwieng W, Prapanthadara LA: A novel F1552/C1552 point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. Pestic Biochem Physiol. 2010, 96: 127-131. 10.1016/j.pestbp.2009.10.005.
Hu Z, Du Y, Nomura Y, Dong K: A sodium channel mutation identified in Aedes aegypti selectively reduces cockroach sodium channel sensitivity to type I, but not type II pyrethroids. Insect Biochem Mol Biol. 2011, 41: 9-13. 10.1016/j.ibmb.2010.09.005.
Chang C, Shen WK, Wang TT, Lin YH, Hsu EL: A novel amino acid substitution in a voltage-gated sodium channel is associated with knockdown resistance to permethrin in Aedes aegypti. Insect Biochem Mol Biol. 2009, 39: 272-278. 10.1016/j.ibmb.2009.01.001.
Brito LP, Linss JG, Lima-Camara TN, Belinato TA, Peixoto AA: Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS One. 2013, 8: e60878-10.1371/journal.pone.0060878.
Zaim M, Aitio A, Nakashima N: Safety of pyrethroid-treated mosquito nets. Med Vet Entomol. 2000, 14: 1-5. 10.1046/j.1365-2915.2000.00211.x.
Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD: The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011, 476: 450-453. 10.1038/nature10355.
Harris AF, Nimmo D, McKemey AR, Kelly N, Scaife S: Field performance of engineered male mosquitoes. Nat Biotechnol. 2011, 29: 1034-1037. 10.1038/nbt.2019.
Acknowledgements
We thank Dr Alexandre Afranio Peixoto for his friendship and orientation throughout this study. This work is dedicated to his memory. We also thank the DNA sequencing facility of FIOCRUZ (Plataforma de Sequenciamento/PDTIS/Fiocruz) and the Brazilian Dengue Control Program that allowed utilization of samples collected in the scope of the Brazilian Aedes aegypti Insecticide Resistance Monitoring Network (MoReNAa). We are grateful to Dr Andrea Gloria-Soria for critical reading the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: JGBL, LPB, AJM. Performed the experiments: JGBL, LPB, GAG. Analyzed the data: JGBL, LPB, ASA, RVB, AJM. Contributed reagents/materials/analysis tools: RVB, JBPL, DV. Wrote the paper: AJM, DV. All authors read and approved the final version of the manuscript.
Luiz Paulo Brito contributed equally to this work.
Electronic supplementary material
13071_2013_1196_MOESM1_ESM.docx
Additional file 1: Table S1:Kdr allele frequencies of Aedes aegypti natural populations from Brazil. The CI95%* is under parentheses. (DOCX 124 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Linss, J.G.B., Brito, L.P., Garcia, G.A. et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasites Vectors 7, 25 (2014). https://doi.org/10.1186/1756-3305-7-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-7-25