Abstract
Background
The clearance of hepatitis C virus infection by interferon therapy significantly reduces the incidence of hepatocellular carcinoma and death in elderly chronic hepatitis patients. However, there are few reports concerning the efficacy and safety of pegylated interferon-alpha2b plus ribavirin combination therapy in elderly patients. The aims of the present study were to examine the effect and safety of pegylated interferon-alpha2b plus ribavirin combination therapy in 427 patients with chronic hepatitis C infection. We compared the rates of sustained virological response--defined as the absence of detectable hepatitis C virus in serum 24 weeks after the treatment ended--and the treatment discontinuation rate between 319 younger patients aged < 65 years and 108 elderly patients aged ≥ 65 years. We also examined the factors contributing to a sustained virological response.
Results
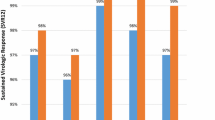
There was no significant difference in the sustained virological response rate between younger patients and elderly patients according to their hepatitis C virus genotype (41.5% (100/241) and 40.7% (35/86) for genotype 1; P = 0.899, 89.7% (70/78) and 86.4% (19/22) for genotype 2; P = 0.703, respectively). There was also no significant difference in the treatment discontinuation rate between the two age groups (10.3% (33/319) and 13.9% (15/108), respectively; P = 0.378). There were no serious adverse events requiring hospitalization. The factors contributing significantly to a sustained virological response in elderly patients were gender, hepatitis C virus genotype, platelet count, and the presence of a rapid or early virological response (undetectable hepatitis C virus in serum at weeks 4 or 12 of treatment, respectively). However, upon multivariate analysis, the presence of an early virological response was the only significant factor (odds ratio: 0.115, 95% confidence interval: 0.040- 0.330, P < 0.001).
Conclusions
The efficacy and safety of pegylated interferon-alpha2b plus ribavirin combination therapy in elderly patients are not always inferior to those in younger patients. Obtaining an early virological response may be essential to achieve a sustained virological response in elderly patients with chronic hepatitis C infection.
Similar content being viewed by others
Background
Chronic hepatitis C virus (HCV) infection affects approximately 300 million people worldwide and is the most common cause of chronic liver disease [1]. Among HCV-infected individuals, 20-30% eventually develop cirrhosis or hepatocellular carcinoma (HCC). In Japan, approximately 30,000 people die of HCC every year, and 70-80% of these deaths are because of HCV infection. Therefore, it is important to eliminate HCV with interferon (IFN) therapy to prevent HCC [2, 3].
Hepatitis C virus entered and expanded in Japan decades before it did so in the United States [4]. In Japan, the patients with chronic HCV who are currently treated with IFN are 10 to 15 years older than the corresponding patients in the United States and Western countries, where the patients treated with antiviral therapy average 45 years of age.
In Japan, HCV infection is characterized by an increasing prevalence with age [5]. Previous studies have reported that clearance of HCV by IFN therapy significantly reduces the incidence of HCC and death in older chronic hepatitis patients [3]. Therefore, it is particularly important to eliminate HCV in elderly patients.
The standard treatment for patients chronically infected with HCV is pegylated interferon (Peg-IFN) and ribavirin combination therapy for 24 or 48 weeks, according to the HCV genotype [6]. This treatment protocol reportedly obtained sustained virological response (SVR) rates-defined as undetectable HCV in serum 24 weeks after the treatment ended--ranging from 45% in patients with HCV genotype 1 to 85% in patients with genotypes 2 or 3 [7, 8]. Recently, the addition of a protease inhibitor to Peg-IFN plus ribavirin combination therapy (triple therapy) has been reported to improve the anti-viral effect [9]. However, these figures were obtained in patients aged 40 to 50 years, as older patients have been excluded from large therapeutic trials [7–9]. Therefore, to date, there are few data concerning the response and safety of this combination treatment in elderly patients [10–12].
The aims of the present study were to examine the effect and safety of Peg-IFN-alpha2b and ribavirin combination therapy in elderly patients and to examine the factors contributing to the SVR in this combination therapy.
Methods
Patients
Between January 2005 and April 2008, 427 consecutive patients with chronic hepatitis C were treated with Peg-IFN-alpha2b and ribavirin combination therapy at the Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, Japan. Each patient was followed up for at least 24 weeks after the treatment ended. Of the 427 patients, 319 were < 65 year old (younger patients) and 108 were ≥ 65 years old (elderly patients). Whether the patients were treated with this combination therapy was mainly based on the decisions of their attending physicians, considering factors such as age, motivation for the combination therapy, general condition, and underlying diseases. Treatment of chronic HCV with a combination of Peg-IFN-alpha2b and ribavirin was approved by the Japanese Ministry of Health in October, 2004. However, the use of PEG-IFN-alpha2a and ribavirin was only approved in September, 2008. Therefore, in the present study, all patients were treated with PEG-IFN-alpha2b and ribavirin combination therapy.
We analyzed the clinical data retrospectively. Patients were excluded if they tested positive for human immunodeficiency virus infection, or if they had decompensated liver disease, liver disease of other cause, psychiatric illness, severe cardiovascular disease, autoimmune disease, poorly controlled diabetes mellitus, renal disease, or alcohol abuse. A liver biopsy was performed in all patients before the treatment, and all biopsy specimens were assigned a fibrosis score and an activity score based on the criteria of Bedossa et al. [13]. This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki, and informed consent was obtained from all patients. We received ethical approval for the present study from the ethics committee of our hospital, Osaka Red Cross Hospital, Japan.
Treatment schedule
Patients were treated with Peg-IFN-alpha2b (1.5 μg/kg/week) plus oral ribavirin according to the Japanese guidelines (total dose 600 mg/day for patients weighing < 60 kg; 800 mg/day for patients with a body weight of 60-80 kg; 1,000 mg/day for patients weighing > 80 kg). Treatment was given for 48 weeks for genotype 1 and 24 weeks for genotype 2. If hematologic side effects were observed, the doses were reduced according to information from the manufacturer. However, in the case of side effects associated with subjective symptoms of Grade 2 or above, such as malaise, fever, anorexia, or light-headedness, the dose of Peg-IFN was reduced by 10-20 μg/week, and the dose of ribavirin was reduced by 200 mg/day as soon as possible, and both were maintained at the lower levels until the severity of the symptoms lessened to Grade 1 or below. During the treatment, we did not use erythropoietin or granulocyte-macrophage colony stimulating factor in any patient.
Virological evaluation
Serum HCV-RNA levels were quantified and qualitatively analyzed using a Cobas Amplicor HCV Monitor Test, version 2.0 (detection range 6-5,000 kIU/ml, lower limit of detection 50 IU/ml; Roche Diagnostics, Branchburg, NJ). HCV genotype was determined by polymerase chain reaction (PCR) amplification of the core region of the HCV genome using genotype-specific PCR primers. [14]
Assessment of treatment efficacy
A rapid virological response (RVR) and an early virological response (EVR) were defined as undetectable serum HCV-RNA at week 4 and week 12 of the therapy, respectively. An end-of-treatment response was defined as undetectable HCV-RNA at the end of the therapy (i.e., week 48 for genotype 1, week 24 for genotype 2). A sustained virological response (SVR) was defined as undetectable HCV-RNA 24 weeks after completion of treatment. All assessment methods were defined according to published guidelines [15, 16]. Even if the treatment was prematurely discontinued because of side effects or non-compliance, patients could be considered to have an SVR on the basis of their serum HCV-RNA results at the 24-week follow-up. Non-response was defined as a lack of HCV-RNA clearance during treatment and at follow-up. During the follow-up period, clinical, biochemical, and qualitative serum HCV-RNA evaluations were performed every 3 months.
Statistical analysis
Variables were compared between groups by the chi-squared test, Fisher's exact probability test, and the Mann-Whitney U test. Group means, presented as mean values ± standard deviations, were compared using the Student's t-test. The influence of various factors on the response to Peg-IFN and ribavirin combination therapy was evaluated by univariate analysis. All variables found to be significant in the univariate analysis were subjected to multivariate analysis using logistic regression. The data were analyzed using SPSS version 9.0 for Microsoft Windows (SPSS, Inc., Chicago, IL). All statistical analyses were based on two-sided hypothesis tests with a significance level of P < 0.05.
Results
Patients
The baseline characteristics of the elderly patients enrolled in the present study are shown in Table 1. The number of genotype 1 and 2 patients was 86 (79.6%) and 22 (20.4%), respectively. No patients with HCV genotype 3 or 4 were included in the present study because there are very few patients with these genotypes in Japan. All the patients had a high viral load (≥ 10 klU/ml), according to the Japanese criteria. The number of patients aged ≥ 70 years was 24 (22.2%). The oldest patient was an 81-year-old male, and he achieved an SVR.
Assessment of treatment response
SVR rates in the younger patients and the elderly patients according to their HCV genotype were 41.5% (100/241) and 40.7% (35/86) for genotype 1 and 89.7% (70/78) and 86.4% (19/22) for genotype 2, respectively. In terms of SVR rates, there were no significant differences between the two age groups according to HCV genotype (P = 0.899 for genotype 1 and P = 0.703 for genotype 2, respectively) (Table 2).
Among the elderly patients, the overall SVR rate was 50% (54/108). An RVR was obtained in 24 patients (10 of genotype 1, 14 of genotype 2), an EVR was obtained in 61 patients (41 of genotype 1, 20 of genotype 2), and an end-of-treatment response was obtained in 89 patients (68 of genotype 1, 21 of genotype 2). The number of patients with a non-response was 19 (18 of genotype 1, 1 of genotype 2). All the patients who demonstrated an RVR achieved an SVR, and 47 (77%) of the 61 patients who obtained an EVR achieved an SVR. Among the 47 patients who did not obtain an EVR, only seven (14.9%) achieved an SVR. According to gender, 33 males (61.1%) and 21 females (38.9%) achieved an SVR. Among the 63 patients who completed at least 80% of the scheduled Peg-IFN dose, 36 (57.1%) achieved an SVR; similarly, among the 63 patients who completed at least 80% of the scheduled ribavirin dose, 36 (57.1%) achieved an SVR. Among the 47 patients who completed at least 80% of the scheduled Peg-IFN-alpha2b dose, ribavirin dose, and scheduled treatment duration (80/80/80 adherence, as mentioned elsewhere [17]), 27 (57.4%) demonstrated an SVR.
Factors contributing to SVR in elderly patients
In our univariate analysis of factors contributing to an SVR in elderly patients, gender (P = 0.021), HCV genotype (1 or 2) (P < 0.001), platelet count (P = 0.003), the presence of an RVR (P < 0.001), and the presence of an EVR (P < 0.001) contributed significantly (Table 3). However, in our multivariate analysis of these five factors by logistic regression, the presence of an EVR was the only significant factor (odds ratio: 0.115, 95% confidence interval: 0.040-0.330, P < 0.001) (Table 4).
Tolerability and adverse events
There was no significant difference in the treatment discontinuation rate between younger patients and elderly patients (10.3% (33/319) and 13.9% (15/108), respectively; P = 0.378) (Table 5). The causes of treatment discontinuation are shown in Table 5. In eight younger patients and four elderly patients, the attending physician decided to discontinue treatment because the viral load had not decreased by week 24 of the treatment. There were no adverse events that required hospitalization.
Discussion
In ageing chronic hepatitis C patients, especially those over 60 years, liver fibrosis is accelerated and the incidence of HCC is increased [18, 19]. Among aged patients, improving the liver fibrosis and decreasing the risk of HCC are mainly achievable by eradication of the hepatitis C virus [2]. Therefore, the primary goal of the IFN therapy in elderly patients should be HCV eradication.
In the present study, there was no significant difference in SVR rate between the two age groups according to HCV genotype. Moreover, there was no significant difference in the treatment discontinuation rate between the two age groups. Our data suggest that Peg-IFN-alpha2b and ribavirin combination therapy is just as tolerable and effective in elderly patients as in younger patients.
The SVR rate and the discontinuation rate of patients aged ≥ 65 years in the present study are better than those of previous studies [20–24]. Oze et al. reported that the Peg-IFN dose affects the virologic response [25], and Hiramatsu et al. reported that ribavirin dose reduction increases the relapse rate [26]. However, in the present study, there were no significant relationships between the total Peg-IFN dose, total ribavirin dose, or treatment duration and SVR. These differences may have arisen because the previous studies were conducted mainly in patients aged ≤ 65 years. In the case of elderly patients, maintaining drug therapy at the standard dose often causes side effects, which makes treatment discontinuation or significant treatment dose reduction inevitable. Oze et al. [24] investigated the efficacy and adverse effects of Peg-IFN plus ribavirin therapy in aged patients with chronic hepatitis C. We compared their SVR and treatment discontinuation rates with ours according to the dose of Peg-IFN and ribavirin. Although Oze et al. [24] used a larger dose of both Peg-IFN and ribavirin than we did (Peg-IFN dose: 1.22 ± 0.32 μg/kg/week (our data) vs. 1.44 ± 0.18 μg/kg/week (Oze et al.); ribavirin dose: 9.58 ± 2.93 mg/kg/day (our data) vs. 11.5 ± 1.7 mg/kg/day (Oze et al.)), the SVR rate was 40.7% in genotype 1 elderly patients in our study and less than 30% in genotype 1 elderly patients in the study by Oze et al. The treatment discontinuation rate in elderly patients was also significantly different between the studies: 13.9% in our study vs. less than 30% in the study by Oze et al. A recent large-scale study demonstrated that the SVR rate did not differ between standard-dose (1.5 μg/kg/week) and low-dose (1.0 μg/kg/week) Peg-IFN-alpha2b therapy [27]. In fact, in elderly patients, dose reduction in a relatively early phase, based on careful monitoring of side effects, may prevent treatment discontinuation and lead to an improved SVR rate.
Sezaki et al. reported that in elderly patients, the response to Peg-IFN and ribavirin combination therapy is worse in female than in male patients [28]. Our data are in agreement with this (SVR of 61.1% in males and 38.9% in females; P = 0.021).
In our multivariate analysis, the presence of an EVR was the only factor contributing significantly to an SVR. Although the positive predictive value for an RVR was 100% (24/24), it was not a significant factor in the multivariate analysis probably because an SVR was achieved without an RVR in many cases. The key to successful IFN therapy in elderly patients is likely to be achieving an EVR. However, if this requires maintaining a high Peg-IFN dose, there is a high possibility that subsequent large dose reduction or even treatment discontinuation will be needed. Thus, careful monitoring is necessary in elderly patients, especially within the first 12 weeks of their treatment. Moreover, if an EVR is not achieved, early discontinuation of treatment may be important in terms of preventing unnecessary treatment and side effects, because, in the present study, among the 47 patients who did not obtain an EVR, only seven (14.9%) achieved an SVR.
This study has several limitations. First, our study is a retrospective study. Second, the number of elderly patients in the present study was low. In our multivariate analysis, the presence of an EVR was the only significant factor contributing to an SVR in elderly patients. However, in general, the four factors that were significant in our univariate analysis (i.e., the presence of an RVR, gender, HCV genotype, and platelet count) are also reported to be significant predictive factors for an SVR [21–26]. Therefore, we should interpret our results with caution, and larger studies are needed. Third, all patients who had serious underlying diseases were excluded from our study. In elderly patients with serious underlying diseases, an ursodeoxycholic acid and/or glycyrrhizin-containing preparation (Stronger Neo-Minophagen C) will be needed when serum alanine aminotransferase levels are higher than the normal upper limit, because these patients would be expected to experience serious side effects from Peg-IFN-alpha2b plus ribavirin combination therapy or other IFN therapy.
Therefore, it is disputable whether the results of our study are applicable to all elderly patients, who often have underlying diseases. These issues should be clarified in future prospective studies.
Conclusion
In conclusion, the effects of Peg-IFN-alpha2b plus ribavirin combination therapy for HCV infection in elderly patients are not always inferior to those in younger patients. Peg-IFN-alpha2b plus ribavirin combination therapy may be safely extended to elderly patients who have no serious underlying disease.
References
Di Bisceglie AM, Hepatitis C: Lancet. 1998, 351: 351-355. 10.1016/S0140-6736(97)07361-3.
Imai Y, Tamura S, Tanaka H, Hiramatsu N, Kiso S, Doi Y, Inada M, Nagase T, Kitada T, Imanaka K, Fukuda K, Takehara T, Kasahara A, Hayashi N: Reduced risk of hepatocellular carcinoma after interferon therapy in aged patients with chronic hepatitis C is limited to sustained viological responders. J Viral Hepat. 2010, 17: 185-191. 10.1111/j.1365-2893.2009.01163.x.
Arase Y, Ikeda K, Suzuki F, Suzuki Y, Saito S, Kobayashi M, Akuta N, Someya T, Koyama R, Hosaka T, Sezaki H, Kobayashi M, Kumada H: Long-term outcome after interferon therapy in elderly patients with chronic hepatitis C. Intervirology. 2007, 50: 16-23. 10.1159/000096308.
Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002, 99: 15584-15589. 10.1073/pnas.242608099.
Okayama A, Stuver SO, Tabor E, Tachibana N, Kohara M, Mueller NE, Tsubouchi H: Incident hepatitis C virus infection in a community-based population in Japan. J Viral Hepat. 2002, 9 (1): 43-51. 10.1046/j.1365-2893.2002.00331.x.
Seeff LB, Hoofnagle JH: Appendix: the national institutes of health consensus development conference management of hepatitis C 2002. Clin Liver Dis. 2003, 7: 261-287. 10.1016/S1089-3261(02)00078-8.
Manns MP, McHutchinson JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001, 358: 958-965. 10.1016/S0140-6736(01)06102-5.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J: Peginteferon alfa-2a plus ribavirn for chronic hepatitis C virus infection. N Engl J Med. 2002, 347: 975-982. 10.1056/NEJMoa020047.
McHutchson JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ: PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Eng J Med. 2009, 360: 1827-1838. 10.1056/NEJMoa0806104.
Huang CF, Yang JF, Dai CY, Huang JF, Hou NJ, Hsieh MY, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL, Yu ML: Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J Infect Dis. 2010, 201: 705-709.
Antonucci G, Longo MA, Angeletti C, Vario F, Oliva A, Comandini UV, Tocci G, Boumis E, Noto P, Solmone MC, Capobianchi MR, Girardi E: The effect of age on response to therapy with peginterferon alpha plus ribavirin in a cohort of patients with chronic HCV hepatitis including subjects older than 65 year. Am J Gastroenterology. 2007, 102: 1383-1391. 10.1111/j.1572-0241.2007.01201.x.
Floreani A, Minola E, Carderi I, Ferrara F, Rizzotto ER, Baldo V: Are elderly patients poor candidates for pegylated interferon plus ribavirin in the treatment of chronic hepatitis C?. J Am Geriatr Soc. 2006, 54: 549-550. 10.1111/j.1532-5415.2006.00643_4.x.
Bedossa P, Poynald T: An algorithm for the grading of activity in chronic hepatitis C. The METAVIR cooperative study group. Hepatology. 1996, 24: 289-293. 10.1002/hep.510240201.
National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: June 10-12, 2002. Hepatology. 2002, 36 (Suppl): S3-20.
Ohno O, Mizokami M, Wu RR, Saleh MG, Ohba K, Orito E, Mukaide M, Williams R, Lau JY: New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997, 35: 201-7.
Strader DB, Wright T, Thomas DL, Seeff LB: American association for the study of liver diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004, 39 (4): 1147-71. 10.1002/hep.20119.
McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ, Albrecht JK: International hepatitis interventional therapy group. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002, 123: 1061-69. 10.1053/gast.2002.35950.
Poynard T, Bedossa P, Opolon P: Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997, 349: 825-832. 10.1016/S0140-6736(96)07642-8.
Hamada H, Yatsuhashi H, Yano K, Daikoku M, Arisawa K, Inoue O, Koga M, Nakata K, Eguchi K, Yano M: Impact of aging on the development of hepatocellular carcinoma in patients with posttransfusion chronic hepatitis C. Cancer. 2002, 95: 331-339. 10.1002/cncr.10662.
Antonucci G, Longo MA, Angeletti C, Vario F, Oliva A, Comandini UV, Tocci G, Boumis E, Noto P, Solmone MC, Capobianchi MR, Girardi E: The effect of age on response to therapy with peginterferon alpha plus ribavirin in a cohort of patients with chronic HCV hepatitis including subjects older than 65 yr. Am J Gastroenterology. 2007, 102: 1383-1391. 10.1111/j.1572-0241.2007.01201.x.
Iwasaki Y, Ikeda H, Araki Y, Osawa T, Kita K, Ando M, Shimoe T, Takaguchi K, Hashimoto N, Kobatake T, Tomita M, Kawaguchi M, Kobashi H, Sakaguchi K, Shiratori Y: Limitation of Combination Therapy of Interferon and Ribavirin for Older Patients With Chronic Hepatitis C. Hepatology. 2006, 43: 54-63. 10.1002/hep.20984.
Honda T, Katano Y, Simizu J, Ishizu Y, Doizaki M, Hayashi K, Ishigami M, Itoh A, Hirooka Y, Nakano I, Urano F, Yoshioka K, Toyoda H, Kumada T, Goto H: Efficacy of peginterferon-α-2b plus ribavirin in patients aged 65 years and older with chronic hepatitis C. Liver Int. 2010, 30: 527-537. 10.1111/j.1478-3231.2009.02064.x.
Edoardo GG, Monica B, Vincenzo S, Antonino P: Predictive factors for response to peginterferon-alpha and ribavirin treatment of chronic HCV infection in patients aged years and more. Dig Dis Sci. 2010, 55: 3193-3199. 10.1007/s10620-010-1408-x.
Oze T, Hiramatsu N, Yakushijin T, Mochizuki K, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, Inui Y, Hijioka T, Inada M, Kaytayama K, Tamura S, Yoshihara H, Inoue A, Imai Y, Kato M, Miyagi T, Yoshida Y, Tatsumi T, Kiso S, Kanto T, Kasahara A, Takehara T, Hayashi N: Indications and limitations for aged patients with chronic hepatitis C in pegylated interferon alfa-2b plus ribavirin combination therapy. J Hepatology. 2011, 54: 604-611. 10.1016/j.jhep.2010.07.043.
Oze T, Hiramatsu N, Yakushijin T, Kurokawa M, Igura T, Mochizuki K, Imanaka K, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Inui Y, Hijioka T, Tamura S, Yoshihara H, Hayashi E, Inoue A, Imai Y, Kato M, Yoshida Y, Tatsumi T, Ohkawa K, Kiso S, Kanto T, Kasahara A, Takehara T, Hayashi N: Pegylated interferon alpha-2b (Peg-IFN alpha-2b) affects early virologic response dose-dependently in patients with chronic hepatitis C genotype 1 during treatment with Peg-IFN alpha-2b plus ribavirin. J Viral Hepat. 2009, 16: 578-585. 10.1111/j.1365-2893.2009.01116.x.
Hiramatsu N, Oze T, Yakushijin T, Inoue Y, Igura T, Mochizuki K, Imanaka K, Kaneko A, Oshita M, Hagiwara H, Mita E, Nagase T, Ito T, Inui Y, Hijioka T, Katayama K, Tamura S, Yoshihara H, Imai Y, Kato M, Yoshida Y, Tatsumi T, Ohkawa K, Kiso S, Kanto T, Kasahara A, Takehara T, Hayashi N: Ribavirin dose reduction raises relapse rate dose-dependently in genotype 1 patients with hepatitis C responding to pegylated interferon alpha-2b plus ribavirin. J Viral Hepat. 2009, 16: 586-595. 10.1111/j.1365-2893.2009.01106.x.
McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS, IDEAL Study Team: Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009, 361: 580-593. 10.1056/NEJMoa0808010.
Sezaki H, Suzuki F, Kawamura Y, Yatsuji H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Miyakawa Y, Kumada H: Poor response to pegylated interferon and ribavirin in older women infected with hepatitis C virus of genotype 1b in high viral loads. Dig Dis Sci. 2009, 54: 1317-1324. 10.1007/s10620-008-0500-y.
Acknowledgements
We would like to thank Haruko Takada for the data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests. None of the authors has had within the past 12 months any financial relationship with a biotechnology manufacturer, a pharmaceutical company, or other commercial entity that has any interest in the subject matter, materials, or processes discussed in the manuscript.
Authors' contributions
YO, TK, SS, AS, and TI participated in the design of the study and performed the statistical analysis; RK helped to draft the manuscript. EI, SA, YK, TI, HT, FM, JN, KH, and SH collected the data. All authors have read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nishikawa, H., Iguchi, E., Koshikawa, Y. et al. The effect of pegylated interferon-alpha2b and ribavirin combination therapy for chronic hepatitis C infection in elderly patients. BMC Res Notes 5, 135 (2012). https://doi.org/10.1186/1756-0500-5-135
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-0500-5-135