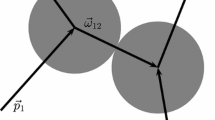
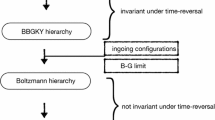
Abstract
Boltzmann’s reply to Loschmidt’s reversibility paradox (1877) has baffled many readers, owing to imprecise language and unproven assumptions. Based on a new translation and detailed commentary, it will be shown that this text nevertheless contains the essentials of a correct, insightful interpretation of thermodynamic irreversibility in statistico-mechanical context.



Similar content being viewed by others
Notes
There already are a nearly complete French translation (Dugas 1959, pp. 188–191) and two complete English translations: Brush (1998, pp. 188–193), Gallavotti (2014, pp. 171–174). Translated extracts and critical comments are found in Klein (1973, pp. 71–74), Brush (1976, pp. 239, 605–607), Sklar (1993, p. 39), von Plato (1994, pp. 87–89), Cercignani (1998, § 5.2), Uffink (2007, § 4.3), Badino (2011, pp. 373–374).
The chief protagonists of this debate are René Dugas, Martin Klein, and Jos Uffink (in favor of radical departure); Jan von Plato, Michel Janssen, and Massimo Badino (in favor of continuity). For references and further discussion, see Darrigol (2018, pp. 542–544).
Loschmidt (1876, p. 139).
Brush: this theorem.
Brush: we must conceive.
Brush: a certain body.
We thereby include all material points of all bodies that interact with the bodies under consideration, directly or indirectly. Strictly speaking, all bodies of the universe ought to be included, because we cannot build a complex of bodies that has strictly no relation to the other bodies of the universe, we can only imagine it.
Needless to say, for a picture [Anschauung] of the mode of action of natural forces in which this [reversal] is not true (as could happen, for instance, in a dynamic picture), the following considerations would also fail to apply.[The reference to this footnote is missing in the original WB publication; Hasenöhrl reintroduced it in BWA, at a location different from the one assumed in this translation]. [By “dynamic picture,” Boltzmann probably means a view, such as Lesage’s, in which the forces between atoms are traced to motion of intermediate matter].
Brush: the sign of this integral …does not depend on the force law.
Brush omits “rightly” (geradezu).
Brush: and.
Brush omits the “more likely”: will become uniform.
Brush: a long time.
Brush omits this last sentence (after the semicolon).
Brush gives a completely different translation of this sentence. In particular, he renders folgen auf as “result in” instead of “ensue from.” So does Dugas, in French.
Brush: It is only in special cases that…
Brush: if one starts with oxygen and hydrogen mixed in a container…
Brush: there is also an individual very improbably initial condition…
Brush: atom.
Brush omits “nearly”.
Brush: If perhaps this reduction of the second law to the realm of probability makes its application to the entire universe appear dubious, yet the laws of probability theory are confirmed by all experiments carried out in the laboratory.
Boltzmann (1872, pp. 345–346).
Uffink (2007, § 4.3.1, Point 1) gives this interpretation of the “sophism” and therefore sees a contradiction with Boltzmann’s subsequent admission of initial states for which the entropy law is violated. Without using the word sophism, Sklar (1993, p. 39) correctly remarks that in Boltzmann’s opinion, Loschmidt’s argument does not force us “to posit the existence of specific initial conditions” and that we can instead “take the statistical viewpoint.”
This is properly noted in Uffink (2007, § 4.3.1, Point 2).
Cf. Uffink (2007, § 4.3.1, Point 4).
Boltzmann 1877b. The alternative meaning of “state-distribution” already occurred in Boltzmann’s memoirs of 1871 and 1872.
As is well known, this calculation is done by imagining a reversible transformation connecting the initial state to the end state and integrating the ratio dQ/T.
In contrast, when we already know that the system has been evolving from a state out of equilibrium, there is only a very small fraction of the microstates compatible with the present macrostate that are compatible with this knowledge, and A1 cannot be true.
Zermelo (1896, pp. 795–796) strongly attacked A2 in 1896, arguing that probabilities by themselves could say nothing on the evolution of a system. Uffink (2007, § 4.3.1, Points 2 and 3) deplores the lack of justification of both A1 and A2, and his overall judgment of Boltzmann’s reply is unflattering: “One may question whether his considerations of the probability of the initial state hit the nail on the head. Probability theory is equally neutral to the direction of time as is mechanics.”
This justification is fragile, because there is no warranty that in actual relaxation experiments all possible initial microstates may occur.
Boltzmann (1868, pp. 95–96; 1881b, pp. 592–593). See Darrigol (2018, pp. 558–560). Boltzmann’s analysis does not apply to systems for which the microcanonical distribution does not yield the observed time-averages. This, the case for systems involving long-range coupling, encountered, for instance, in plasma physics. For an extension of statistical mechanics to such systems, cf. Tsallis 2009.
In such experiments there is a common arrow of time, from the unlifted to the lifted sliding wall. In other words, the temporal asymmetry, which is absent in the complete H curve, is generated by the preparation of the initial state.
Eddington (1929, p. 68).
The Ehrenfest (1911, pp. 44–45) gave a similar interpretation of some of Boltzmann’s remarks, based on the “concentration curve” (Verdichtungskurve) for the bundle of H-curves starting from the microstates compatible with the given initial distribution. See Sklar (1993, pp. 63–67), Uffink (2007, pp. 60–61).
Boltzmann 1896–1898, vol. 2, p. 112. Cf. Darrigol (2018, p. 443).
References
Alder, Berni, and Thomas Wainwright 1960 Studies in molecular dynamics. II. Behavior of a small number of elastic spheres. Journal of chemical physics, 33: 1439–1451.
Ardourel, Vincent 2017 Irreversibility in the derivation of the Boltzmann Equation. Foundations of physics, 47: 471–489.
Badino, Massimiliano 2011 Mechanistic slumber vs. statistical insomnia: The early history of Boltzmann’s H-theorem (1868–1877). The European physical journal H, 36: 353–378.
Boltzmann, Ludwig 1866 Über die mechanische Bedeutung des zweiten Hauptsatzes der Wärmetheorie. WB, 53: 195-220. Also in BWA1, 9–33.
Boltzmann, Ludwig 1868 Studien über das Gleichgewicht der lebendigen Kraft zwischen bewegten materiellen Punkten. WB, 58: 517–560. Also in BWA1, 49–96.
Boltzmann, Ludwig 1871 Analytischer Beweis des zweiten Hauptsatzes der mechanischen Wärmetheorie aus den Sätzen über das Gleichgewicht der lebendigen Kraft. WB, 63: 712–732. Also in BWA1, 288–308.
Boltzmann, Ludwig 1872 Weitere Studien über das Wärmegleichgewicht unter Gasmolekülen. WB, 66: 275–370. Also in BWA1, 316–402.
Boltzmann, Ludwig 1877a Bemerkungen über einige Probleme der mechanischen Wärmetheorie. WB, 7: 62–100. Also in BWA2, 112–148.
Boltzmann, Ludwig 1877b Über die Beziehung zwischen dem zweiten Hauptsatze der mechanischen Wärmetheorie und der Wahrscheinlichkeitsrechnung respektive den Sätzen über das Wärmegleichgewicht. WB, 76: 373–435. Also in BWA2, 164–223.
Boltzmann, Ludwig 1881a Über einige das Wärmegleichgewicht betreffende Sätze. WB, 84: 136–145. Also in BWA2, 572–581.
Boltzmann, Ludwig 1881b [Review of Maxwell 1879]. In Beiblätter zu Annalen der Physik, 5: 403–417. English in Philosophical magazine, 14 (1882), 299–313. Also in BWA2, 582–595.
Boltzmann, Ludwig 1895 On certain questions of the theory of gases. Nature, 51: 413–415. Also in BWA3, 535–544.
Boltzmann, Ludwig 1896–1898 Vorlesungen über die Gatheorie. 2 vols. Leipzig: Barth.
Boltzmann, Ludwig 1897 Zu Hrn. Zermelos Abhandlung ’über die mechanische Erklärung irreversibler Vorgänge.’ Annalen der Physik, 60: 392–398. Also in BWA3, 579–586.
Boltzmann, Ludwig 1898 Über die sogenannte H-Kurve, Mathematische Annalen, 50: 325–332. Also in BWA3, 629–637.
Brown, Harvey, Wayne Myrvold, and Jos Uffink 2009 Boltzmann’s H-theorem, its discontents, and the birth of statistical mechanics. Studies in the history and philosophy of modern physics, 40: 174–191.
Brush, Stephen 1966 Kinetic theory. Vol. 2: Irreversible processes. New York: Pergamon Press.
Brush, Stephen 1976 The kind of motion we call heat: A history of the kinetic theory of gases in the 19th century, 2 vols. Amsterdam.
Cercignani, Carlo 1998 Ludwig Boltzmann: The man who trusted atoms. Oxford: Oxford University Press.
Darrigol, Olivier 2018 Atoms, mechanics, and probability: Ludwig Boltzmann’s statistico-mechanical writings—an exegesis. Oxford: Oxford University Press.
Darrigol, Olivier 2021 Paperback edition of the former, including a few corrections.
Dugas, René 1959 La théorie physique au sens de Boltzmann. Neuchâtel: Griffon.
Eddington, Arthur Stanley 1929 The nature of the physical world [The Gifford lectures of 1927, Edinburgh]. New York: MacMillan.
Ehrenfest, Paul, and Tatiana Ehrenfest 1911 Begriffliche Grundlagen der statistischen Auffassung in der Mechanik. Encyklopädie der mathematischen Wissenschaften, IV/2.II: 1–90.
Gallavotti, Giovanni 2014 Nonequilibrium and irreversibility. Heidelberg: Springer.
Garber, Elisabeth, Stephen Brush, and C.W.F. Everitt 1995 Maxwell on heat and statistical mechanics. Bethlehem: Lehigh University Press.
Hahn, Erwin 1950 Spin echoes. Physical review, 80: 580–594.
Klein, Martin 1970 Maxwell, his demon, and the second law of thermodynamics. American scientist, 58: 84–97.
Klein, Martin 1973. The development of Boltzmann’s statistical ideas. In Ezechiel Godert David Cohen and Walter Thirring (eds.), The Boltzmann equation: Theory and applications (Vienna: Springer), 53–106.
Loschmidt, Josef 1876 Über den Zustand des Wärmegleichgewichtes eines Sytsems von Körpern mit Rücksicht auf die Schwerkraft. I. WB, 73: 128–142.
Orban, John, and André Bellemans 1967 Velocity inversion and irreversibility in a dilute gas of hard disks. Physics letters, 24A: 620–621.
Schrödinger, Erwin 1951 Irreversibility. Proceedings of the Royal Irish Academy, 53: 189–195.
Sklar, Lawrence 1993 Physics and chance: Philosophical issues in the foundations of statistical mechanics. Cambridge: Cambridge University Press.
Tsallis, Constantino 2009 Introduction to nonextensive statistical mechanics: Approaching a complex world. New York: Springer.
Uffink, Jos 2007 Compendium to the foundations of classical statistical mechanics. In Jeremy Butterfield and John Earman (eds.) Handbook for the philosophy of physics (Amsterdam: Elsevier), 924–1074.
Uffink, Jos, and Giovanni Valente 2015 Lanford’s theorem and the emergence of irreversibility. Foundations of physics, 45: 404–438.
von Plato, Jan 1994 Creating modern probability: Its mathematics, physics and philosophy in historical perspective. Cambridge: Cambridge University Press.
Watson, Henry William 1876 A treatise on the kinetic theory of gases. Oxford: Clarendon Press.
Waugh, John, Won-Kyu Rhim, and Alexander Pines 1972 Spin echoes and Loschmidt’s paradox. Journal of pure and applied chemistry, 32: 317–324.
Zermelo, Ernst 1896 Über mechanische Erklärungen irreversibler Vorgänge. Eine Antwort auf Hrn. Boltzmann’s ‘Entgegnung’. Annalen der Physik, 59: 793–801.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darrigol, O. Boltzmann’s reply to the Loschmidt paradox: a commented translation. EPJ H 46, 29 (2021). https://doi.org/10.1140/epjh/s13129-021-00029-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjh/s13129-021-00029-2