Abstract
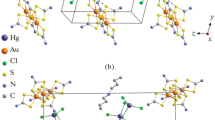
Chemisorption of gold(III) from solutions in 2 M HCl with freshly precipitated binuclear zinc dithiocarbamate [Zn2{S2CN(C4H9)2}4] resulted in the formation of a polymeric heteronuclear gold(III)–zinc(II) dithiocarbamato-chlorido complex ([Au{S2CN(C4H9)2}2]2[ZnCl4]) n (I), which was characterized by MAS 13C NMR, X-ray diffraction (CIF file CCDC no. 1526616), and simultaneous thermal analysis. Compound I isolated on a preparative scale was found to have a highly intricate supramolecular structure composed of 13 centrosymmetric and non-centrosymmetric isomeric complex cations, [Au{S2CN(C4H9)2}2]+, with 24 structurally non-equivalent BuDtc ligands, and six isomeric [ZnCl4]2– anions. The isomeric gold(III) cations perform different structural functions. Four and six cations are involved in the formation of two sorts of long-period cation–cationic chains (via pair non-valence secondary Au···S bonds): (···A···B···C···D···C···B···) n and (···F···G···H···I···J···K···) n . The discrete E, L, and M cations and the [ZnCl4]2– complex anions are located alongside of the polymer chains and do not take part in the secondary interactions. According to simultaneous thermal analysis, thermolysis of I includes destruction of the dithiocarbamate moiety with reduction of gold to the metal in the cation and liberation of zinc chloride with partial conversion to ZnS in the anion.
Similar content being viewed by others
References
Deb, M.K., Chakravarty, S., and Mishra, R.K., J. Indian. Chem. Soc., 1996, vol. 73, no. 10, p. 551.
Nieuwenhuizen, P.J., Appl. Catal., A, 2001, vol. 207, nos 1-2, p. 55.
Cicotti, M., in Compound Class: Alkylenebis(dithiocarbamates): Handbook of Residue Analytical Methods for Agrochemicals, Lee, P.W., Ed., Chichester: Wiley, 2003, vol. 2, p. 1089.
Onwudiwe, D.C., Nthwane, Y.B., Ekennia, A.C., and Hosten, E., Inorg. Chim. Acta, 2016, vol. 447, p. 134.
Bozdag, M., Carta, F., Vullo, D., et al., Bioorg. Med. Chem., 2015, vol. 23, no. 10, p. 2368.
Carta, F., Aggarval, M., Maresca, A., et al., J. Med. Chem., 2012, vol. 55, no. 4, p. 1721.
Zia-ur-Rehman, Ibrahim, S., Khan, A., et al., J. Coord. Chem., 2016, vol. 69, no. 3, p. 1.
Gomathi, G., Sathiyaraj, E., Thirumaran, S., and Ciattini, S., J. Sulfur Chem., 2016, vol. 37, no. 1, p. 23.
Bharti, A., Bharati, P., Chaudhari, U.K., et al., Polyhedron, 2015, vol. 85, p. 712.
Yadav, R., Trivedi, M., Kociok-Köhn, G., et al., Eur. J. Inorg. Chem., 2016, no. 7, p. 1013.
Hrubaru, M., Onwudiwe, D.C., and Hosten, E., J. Sulfur Chem., 2015, vol. 37, no. 1, p. 37.
Abdullah, N.H., Zainal, Z., Silong, S., et al., Mater. Chem. Phys., 2016, vol. 173, p. 33.
Prakasam, B.A., Lahtinen, M., Peuronen, A., et al., Mater. Lett., 2015, vol. 44, p. 19.
Loseva, O.V., Rodina, T.A., and Ivanov, A.V., Russ. J. Coord. Chem., 2013, vol. 39, no. 6, p. 463.
Ivanov, A.V., Rodina, T.A., and Loseva, O.V., Russ. J. Coord. Chem., 2014, vol. 40, no. 12, p. 875.
Ivanov, A.V., Loseva, O.V., Rodina, T.A., et al., Russ. J. Inorg. Chem., 2014, vol. 59, no. 8, p. 807.
Loseva, O.V. and Ivanov, A.V., Russ. J. Inorg. Chem., 2014, vol. 59, no. 12, p. 1491.
Loseva, O.V., Rodina, T.A., Smolentsev, A.I., and Ivanov, A.V., J. Struct. Chem., 2014, vol. 55, no. 5, p. 901.
Rodina, T.A., Loseva, O.V., Smolentsev, A.I., and Ivanov, A.V., J. Struct. Chem., 2016, vol. 42, no. 1, p. 146.
Byr’ko, V.M., Ditiokarbamaty (Dithiocarbamates), Moscow: Nauka, 1984.
Ivanov, A.V., Ivakhnenko, E.V., Gerasimenko, A.V., and Forsling, W., Russ. J. Inorg. Chem., 2003, vol. 48, no. 1, p. 45.
Zhong, Y., Zhang, W.G., Zhang, Q.J., et al., Acta Chim. Sin., 2003, vol. 61, no. 11, p. 1828.
Klug, H.P., Acta Crystallogr., 1966, vol. 21, no. 4, p. 536.
Bonamico, M., Mazzone, G., Vaciago, A., and Zambonelly, L., Acta Crystallogr., 1965, vol. 19, no. 6, p. 898.
Sreehari, N., Varghese, B., and Manoharan, P.T., Inorg. Chem., 1990, vol. 29, no. 20, p. 4011.
Miyamae, H., Ito, M., and Iwasaki, H., Acta Crystallogr., Sect. B: Struct. Crystallogr., Cryst. Chem., 1979, vol. 35, p. 1480.
Kellö, E., Vrabel, V., Kettmann, V., and Garaj, J., Collect. Czech. Chem. Commun., 1983, vol. 48, p. 1272.
Francetič, V. and Leban, I., Vestn. Slov. Kem. Drus., 1979, vol. 26, p. 113.
Agre, V.M. and Shugam, E.A., Zh. Strukt. Khim., 1972, vol. 13, no. 4, p. 660.
Motevalli, M., O’Brien, P., Walsh, J.R., and Watson, I.M., Polyhedron, 1996, vol. 15, no. 16, p. 2801.
Cox, M.J. and Tiekink, E.R.T., Z. Kristallogr., 1999, vol. 214, no. 3, p. 184.
Tiekink, E.R.T., CrystEngComm, 2003, vol. 5, no. 21, p. 101.
Ivanov, A.V., Korneeva, E.V., Gerasimenko, A.V., and Forsling, W., Russ. J. Coord. Chem., 2005, vol. 31, no. 10, p. 695.
Ivanov, A.V. and Antzutkin, O.N., Topics Curr. Chem., 2005, vol. 246, p. 271.
Shaheen, F., Gieck, C., Badshah, A., and Khosa, M.K., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, vol. 62, no. 6, p. m1186.
Shahid, M., Ruffer, T., Lang, H., et al., J. Coord. Chem., 2009, vol. 62, no. 3, p. 440.
Ferreira, I.P., de Lima, G.M., Paniago, E.B., et al., Inorg. Chim. Acta, 2016, vol. 441, p. 137.
Pines, A., Gibby, M.G., and Waugh, J.S., J. Chem. Phys., 1972, vol. 56, no. 4, p. 1776.
APEX2 (version 1.08), SAINT (version 7.03), SADABS (version 2.11) and SHELXTL (version 6.12), Madison: Bruker AXS Inc., 2004.
Pauling, L., The Nature of the Chemical Bond and the Structure of Molecules and Crystals, London: Cornell Univ., 1960.
Bondi, A., J. Phys. Chem., 1964, vol. 68, no. 3, p. 441.
Bondi, A., J. Phys. Chem., 1966, vol. 70, no. 9, p. 3006.
Exarchos, G., Robinson, S.D., and Steed, J.W., Polyhedron, 2001, vol. 20, nos. 24–25, p. 2951.
Karâa, N., Hamdi, B., Ben Salah, A., and Zouari, R., J. Mol. Struct., 2013, vol. 1049, nos. 1–3, p. 48.
Alcock, N.W., Adv. Inorg. Chem. Radiochem., 1972, vol. 15, no. 1, p. 1.
Razuvaev, G.A., Almazov, G.V., Domrachev, G.A., et al., Dokl. Akad. Nauk SSSR, 1987, vol. 294, no. 1, p. 141.
Lidin, R.A., Andreeva, L.L., and Molochko, V.A., Spravochnik po neorganicheskoi khimii (Handbook in Inorganic Chemistry), Moscow: Khimiya, 1987.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Ivanov, O.V. Loseva, T.A. Rodina, A.I. Smolentsev, 2017, published in Koordinatsionnaya Khimiya, 2017, Vol. 43, No. 8, pp. 482–495.
Rights and permissions
About this article
Cite this article
Ivanov, A.V., Loseva, O.V., Rodina, T.A. et al. Multiple isomerization of structural units in ion-polymeric heteronuclear gold(III)–zinc(II) complex ([Au{S2CN(C4H9)2}2]2[ZnCl4])n: Chemisorption-based synthesis, supramolecular structure (self-organization of long-period cation–cationic polymer chains), and thermal behavior. Russ J Coord Chem 43, 512–525 (2017). https://doi.org/10.1134/S1070328417080036
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328417080036