Abstract
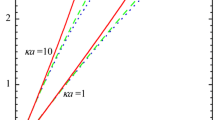
In scope of the Poisson–Boltzmann theory, the electrostatic potential profiles in the vicinity of spherical particles immersed in 1 : 1 electrolyte solutions have been precisely calculated. Using the data on the behavior of the profiles at large distances from the particle surface, effective surface potential \({{\psi }_{{{\text{eff}}}}},\) and its limiting value \(\psi _{{{\text{eff}}}}^{{{\text{sat}}}},\) to which it tends upon an infinite growth of the surface charge, have been determined for a wide range of model parameters (surface charge density, particle radius, and electrolyte concentration). A universal curve has been plotted to represent the dependence of \(\psi _{{{\text{eff}}}}^{{{\text{sat}}}}\) on reduced particle radius κa (a is the radius and κ is the reciprocal Debye screening radius) and to evidently illustrate the existence of two known limiting laws of variations in the effective potential that corresponds to the saturation conditions. The energy criterion and the analysis of its sensitivity to the cutoff threshold have been employed to evaluate the thicknesses of the shells formed by immobilized counterions around the spherical particles. Dependences of the shell thickness on the surface charge density, particle radius, and 1 : 1 electrolyte concentration have been analyzed. It has been revealed that there is limiting thickness \(l_{{{\text{eff}}}}^{{{\text{sat}}}},\) which is reached upon the infinite growth of the surface charge density. A universal κ\(l_{{{\text{eff}}}}^{{{\text{sat}}}}\)(κa) curve is presented and compared with the \(\psi _{{{\text{eff}}}}^{{{\text{sat}}}}\)(κa) curve.








Similar content being viewed by others
REFERENCES
Dolinnyi, A.I., Colloid J., 2019, vol. 81, p. 642.
Verwey, E.J.W. and Overbeek, J.Th.G., Theory of the Stability of Lyophobic Colloids, New York: Elsevier, 1948.
Delahay, P., Double Layer and Electrode Kinetics, New York, 1965; M.: Mir, 1967.
Derjaguin, B.V., Churaev, N.V., and Muller, V.M., Surface Forces, New York: Consultants Bureau, 1987.
Andelman, D., Handbook of Biological Physics, Lipowsky, R. and Sackmann, E., Eds., Elsevier Science, 1995, vol. 1.
Levin, Y., Rep. Prog. Phys., 2002, vol. 65, p. 1577.
Lyklema, J., Fundamentals of Interface and Colloid Science, Amsterdam: Elsevier Academic, 2005, vol. 4, Chap. 3.
Ohshima, H., Nanolayer Research: Methodology and Technology for Green Chemistry, Elsevier, 2017, Chap. 2.
Attard, P., J. Phys. Chem., 1995, vol. 99, p. 14 174.
Bocquet, L., Trizac, E., and Aubouy, M., J. Chem. Phys., 2002, vol. 117, p. 8138.
Aubouy, M., Trizac, E., and Bocquet, L., J. Phys. A, 2003, vol. 36, p. 5835.
Manning, G.S., J. Phys. Chem. B, 2007, vol. 111, p. 8554.
Tellez, G. and Trizac, E., Phys. Rev. E: Stat.Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2003, vol. 68. Article 061401.
González-Mozuelos, P., Guerrero-García, G.I., and Olvera De la Cruz, M., J. Chem. Phys., 2013, vol. 139, Article 064 709.
Guerrero-Garcıa, G.I., Gonzalez-Mozuelos, P., and Olvera De la Cruz, M., ACS Nano, 2013, vol. 7, p. 9714.
Fagotti, B.C., Degiorgio, V., and Piazza, R., Langmuir, 1991, vol. 7, p. 824.
Roberts, J.M., O’Dea, J.J., and Osteryoung, J.G., Anal. Chem., 1998, vol. 70, p. 3667.
Huang, Q.R., Dubin, P.L., Moorefield, C.N., and Newkome, G.R., J. Phys. Chem. B, 2000, vol. 104, p. 898.
Quesada-Perez, M., Callejas-Fernandez, J., and Hidalgo-Alvarez, R., J. Colloid Interface Sci., 2001, vol. 233, p. 280.
Wette, P., Schöpe, H.J., and Palberg, T., J. Chem. Phys., 2002, vol. 116, p. 10 981.
Aswal, V.K. and Goyal, P.S., Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2003, vol. 67, Article 051 401.
Garbow, N., Evers, M., Palberg, T., and Okubo, T., J. Phys. B: Condens. Matter, 2004, vol. 16, p. 3835.
Hsiao, C.C., Wang, T.-Y., and Tsao, H.-K., J. Chem. Phys., 2005, vol. 122, Article 144 702.
Guo, X., Kirton, G.F., and Dubin, P.L., J. Phys. Chem. B, 2016, vol. 110, p. 20 815.
Strubbe, F., Beunis, F., and Neyts, K., J. Colloid Interface Sci., 2006, vol. 301, p. 302.
Gillespie, D.A.J., Hallett, J.E., Elujoba, O., Ham-zah, A.F.C., Richardson, R.M., and Bartlett, P., Soft Matter, 2014, vol. 10, p. 566.
Xu, X., Ran, Q., Haag, R., Ballauff, M., and Dzubiella, J., Macromolecules, 2017, vol. 50, p. 4759.
Trefalt, G., Palberg, T., and Borkovec, M., Curr. Opin. Colloid Interface Sci., 2017, vol. 27, p. 9.
Zimm, B.H. and Le Bret, M., J. Biomol. Struct. Dyn., 1983, vol. 1, p. 461.
Alexander, S., Chaikin, P.M., Grant, P., Morales, G.J., Pincus, P., and Hone, D., J. Chem. Phys., 1984, vol. 80, p. 5776.
Ramanathan, G.V., J. Chem. Phys., 1988, vol. 88, p. 3887.
Belloni, L., Colloids Surf. A, 1998, vol. 140, p. 227.
Schmitz, K.S., Langmuir, 2000, vol. 16, p. 2115.
Sanghiran, V. and Schmitz, K.S., Langmuir, 2000, vol. 16, p. 7566.
Lukatsky, D.B. and Safran, S.A., Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, vol. 63, Article 011 405.
Mukherjee, A.K., Schmitz, K.S., and Bhuiyan, L.B., Langmuir, 2002, vol. 18, p. 4210.
Dufrêche, J.-F., White, T.O., and Hansen, J.-P., Mol. Phys., 2003, vol. 101, p. 1741.
Trizac, E., Bocquet, L., Aubouy, M., and von Grünberg, H.H., Langmuir, 2003, vol. 19, p. 4027.
Diehl, A. and Levin, Y., J. Chem. Phys., 2004, vol. 121, p. 12 100.
Denton, A.R., J. Phys.: Condens. Matter, 2008, vol. 20, Article 494 230.
Lamm, G. and Pack, G.R., Biopolymers, 2010, vol. 93, p. 619.
Carnal, F. and Stoll, S., J. Phys. Chem. A, 2012, vol. 116, p. 6600.
Šamaj, L. and Trizac, E., J. Phys. A, 2015, vol. 48, Article 265 003.
Funding
This work was performed within the framework of a state order to the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Dolinnyi, A.I. Effective Parameters of Charged Spherical Particles in 1 : 1 Electrolyte Solutions. Colloid J 82, 661–671 (2020). https://doi.org/10.1134/S1061933X20060034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X20060034