Abstract
Results of studying pollen emission into the atmosphere are presented for 26 anemophilous and three entomophilous plant species for which optional anemophily is possible. The percentage of clusters of two or more pollen grains of the total number of pollen particles entering the atmosphere is estimated. It is shown that such clusters were formed in significant quantities in all series of experiments. The percentage of pollen clusters reaches ∼71% of the total number of pollen particles. The percentage of pollen grains in the composition of the clusters reaches ∼94% of the total number of pollen grains.
Similar content being viewed by others
INTRODUCTION
Pollen of anemophilous plants is an indispensable component of the coarse fraction of atmospheric aerosol. Its propagation in the atmosphere is a physical process necessary for seed reproduction of anemophilous plants, the main producers of extratropical land biomass. Pollen grains (PGs) cause outbreaks of allergic reactions in 30% of the population [1] and take part in the transfer of chemical elements in biocenoses.
The high allergenicity of pollen has led to the creation of many aero-palynological monitoring stations throughout the world. They are often combined into regional and national networks where long-term observations are carried out by standard techniques. In 2016, there were 879 stations of pollen aerosol sampling in the world: 9 in Africa, 151 in America, 182 in Asia (143 in Japan), 525 in Europe, and 12 in Oceania [2]. In Russia, there are nine stations: in Moscow, St. Petersburg, Yekaterinburg, Krasnodar, Perm, Rostov-on-Don, Ryazan, Stavropol, and Tyumen (https://allergotop.com).
Most often, pollen concentration in the atmosphere is determined using Hirst, Burkard, and Lanzoni slit aspiration traps (>600, or 70% of the stations). Features of pollen particle (PP) morphology (large size, the mean diameter of PG of anemophilous plants is 20–40 µm, drying deformation, and the presence of clusters) are caused by significant difficulties in the process of pollen aerosol (PA) entrapment. The sampling is nonisokinetic, PP settling on the walls of collecting devices is observed [3, 4], the sampling is accompanied by destruction of clusters [5], and the efficiency of trapping of >10-µm particles is unsatisfactory [6] (for example, ∼15% of cereal pollen is not trapped). The analysis of samples collected by impactors requires qualified executives and takes from three to ten days. The error of pollen concentration measurements in air is ∼30% [7]. The currently available technique of PA sampling did not provide continuous monitoring during the COVID-19 pandemic [8]. The quarantine and isolation of personnel prevented observations from being carried out. It is also necessary to note that collections of PA samples allow one to judge the pollen content in the atmosphere only in the vicinity of an observation site. Even in Europe (for example, in Bavaria) the number of pollen monitoring stations is insufficient for forecasting the pollen transfer in the atmosphere [2].
Currently, the AutoPollen program is operative in Europe. It is aimed at creating a prototype of a network of fully automatic pollen monitoring stations. To cover main bioclimatic zones of Europe, it is planned to additionally deploy 200–300 automatic stations allowing one to obtain data in the real time mode (several minutes after sampling). It is believed that timely acquisition of information about taxonomic affiliation, terms of entering, and concentration of PPs in the atmosphere will notably reduce the direct and indirect health costs associated with allergy, currently estimated between €50–150 billion/year [8].
The area of Russia, three times the area of Europe, with a much lower population density and an extremely uneven distribution of the population, objectively hinders the creation of a network of pollen monitoring stations. Models of pollen transport created allowing for vegetation features, pollen production of plants, and processes of PP propagation seem to be more promising for forecasting the pollen content in the atmosphere.
The efficiency of the wind blowing PPs off the anther surface, the time of their stay in the air, distance of transport, and efficiency of capture by collecting surfaces depend on the sedimentation rate.
The PGs of anemophilous plants entering the atmosphere dry out and change their size, shape, and density of the protoplast [9, 10]. In the cytoplasm of a PG vegetative cell, large air spaces can appear [11]. The PG sedimentation rate is most strongly affected by the formation of clusters of two and more PGs [12], the process which is still poorly studied.
This work continues the studies [13–15] of the PA cluster composition during atmospheric pollen emission from 29 kinds of species of plants growing in the Central Siberian Botanical Garden (CSBG), Siberian Branch, Russian Academy of Sciences.
MATERIALS AND METHODS
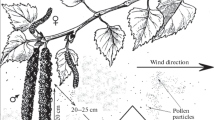
All experiments were carried out in field conditions. We studied the cluster composition of PA entering the atmosphere from plants growing outdoors, both in natural populations and in collection areas of the CSBG. PPs were blown by wind from plant inflorescences to substrates covered with glycerin gelatin with addition of Coomassie blue (Fig. 1). The gust speed was measured by an anemometer and was 0.3–2.0 m/s. The substrates were arranged in the direction of the wind. The distance from the substrates was 20–25 cm, which made it possible to trap a sufficient amount of PPs and to avoid the contact of the substrates with inflorescences. Pollen of each species was sampled five times with intervals of several minutes. The exposure of the substrates lasted 1–2 s. Temperature and relative air humidity were simultaneously measured with a Center 311 device. The pollen particles (individual PGs and their clusters) were counted on ten transects at 10–40-fold magnification of the MBI-11U42 microscope lens.
Features of the PG morphology of anemophilous plants (the pollen is dry, with a thin and smooth outer shell (exine)) impede their coalescence; however, it is not clear how efficiently they interfere with cluster formation. It is supposed that individual PGs enter the atmosphere during the emission from anthers and the observed clusters are formed in the process of sedimentation directly on the microscope slide (Figs. 2 and 3).
The number of clusters of two and more PGs that would be formed on a substrate was estimated under the following assumptions: (i) sedimentation of PGs onto a substrate does not depend on sedimentation of other PGs and (ii) PGs are arranged in a layer in a forming cluster. With an increase in the number of PGs in clusters, their number on a substrate should decrease. If the mathematical expectation of the number of clusters consisting of an arbitrary number of PGs is less than unity, then similar (and larger) PPs are not formed on a substrate at a given number of PGs per unit area.
A cluster is formed if the distance between the geometric centers of PGs does not exceed two radii. Thus, the mathematical expectation of the number of clusters including two or more PGs (N≥ 2) can be represented in the form
where N≥ 1 is the number of PGs in clusters of one and more PGs (in fact, the total number of PGs settled on the substrates);
is the fraction of the substrate surface occupied by PGs (see Fig. 1), Spg is the average area of the PG projection, and ST is the area of the substrate examined.
To estimate the area of individual PGs, photographs of ∼200 PGs of all examined plant species were taken in the Common Use Center for Microscopic Analysis of Biological Objects of the Siberian Branch, Russian Academy of Sciences. The areas of PG projections in the photographs were determined from image processing with the MapInfo Professional software.
The mathematical expectation of the number of clusters of two PGs can be estimated by the relationship
where N≥3 is the mathematical expectation of the number of clusters consisting of at least three PGs,
In the general case, mathematical expectations of the number of clusters of j and more PGs can be represented in the form
The mathematical expectation of the number of individual PGs
In the process of PG settling on a substrate, two alternative variants are possible: PGs appear on the substrate either individually or as components of clusters of two or more PGs. Therefore, the problem can be reduced to comparison of fractions of individual PGs of the total number of PGs settled on the substrates, i.e., to estimation of the significance of differences between fractions or percentages of the feature characterized by an alternative distribution. For this purpose, the Fisher criterion F with the φ-transformation (Fisher angular transformation) was used. It is intended for comparison of two samples according to the frequency of occurrence of an index a researcher is interested in:
where φ1 and φ2 are the transformed fractions and Na ≥ 1, Nb ≥ 1 are the sample volumes (the total numbers of PGs on the substrates). The value obtained was compared with the tabular one at a given level of significance and the number of the degrees of freedom df1 = 1; df2 = Na ≥ 1 + Nb ≥ 2 − 2.
When the sample volumes number in hundreds and thousands, F = 3.8 at the significance level α = 0.05, 6.6 at α = 0.01, and 10.8 at α = 0.001. If the calculated values of F exceed the abovementioned values, the null hypothesis at a given level of significance should be rejected.
RESULTS
Emission of pollen entering the atmosphere directly from anthers of 29 plant species growing in the CSBG, including 26 anemophilous plant species: seven species of woody plants, 14 species of grasses, and five species of weeds (herbs) have been studied. The data about the presence of clusters in the pollen of the plants under study are shown in Table 1. Clusters were revealed in pollen samples of all plants under study. The percentage of clusters of two and more PGs reached 71% of the total number of PPs (Alnus hirsuta); the PG percentage in their composition was 93.7% (Populus alba) of the total number of trapped PGs.
The results of the statistical analysis of plant samples are presented in Table 2.
The Fisher criterion varied from 16.9 (Cyperus papyrus) to 208 (Populus alba), which is obviously higher than its value even at a significance level of 0.001 (10.8). The null hypothesis about formation of clusters from individual PGs on the substrates should be rejected.
Pollen of entomophilous plants significantly differed in its cluster composition (α = 0.001) from the PA produced by anemophilous plants. For example, comparison of percentages of individual PGs produced by Oxalis acetosella and Chosenia arbutifolia yields F = 42.3.
CONCLUSIONS
Results of studying the pollen entering the atmosphere for ∼1/12 of the total number (417) of species represented in the flora of Akademgorodok, Novosibirsk, corroborate preliminary conclusions about the character of the pollen emission [9–11]. The anemophilous plant pollen entering the atmosphere is not monodisperse. In addition to individual PGs, clusters containing two and more PGs enter the atmosphere. The percentage of such clusters of the total number of forming particles varies over a wide range and can be markedly different in different plant species.
The following conclusions can be drawn from the work:
(1) Morphological features of the structure of pollen grains of anemophilous plants do not prevent the formation of clusters in the process of pollen emission into the atmosphere.
(2) Anemophilous plant pollen entering the atmosphere is not monodisperse but represented both by individual PGs and by clusters of two and more PGs.
(3) The fraction of clusters of the total number of particles formed and the percentage of PGs in their composition vary over a wide range and can reach 71 and 93%, respectively.
(4) Anemophilous plant pollen entering the atmosphere significantly differs from pollen of entomophilous plants in its cluster composition.
REFERENCES
T. Biedermann, L. Winther, S. J. Till, P. Panzner, A. Knulst, and E. Valovirta, “Birch pollen allergy in Europe,” Allergy 74 (7), 1237–1248 (2019).
J. T. M. Buters, C. Antunes, A. Galveias, K. C. Bergmann, M. Thibaudon, C. Galan, C. Schmidt-Weber, and J. Oteros, “Pollen and spore monitoring in the world,” Clin. Transl. Allergy 8 (9) (2018). https://doi.org/10.1186/s13601-018-0197-8
B. Crook, “Inertial samplers: Biological perspectives,” in Bioaerosols (Lewis Publishers, Boca Raton, FL, 1995).
B. Crook, “Non-inertial samplers: Biological perspectives,” in Bioaerosols Handbook, Ed. by C.S. Cox and C.M. Wathes (Lewis Publishers, Boca Raton, FL, 1995).
N. A. Fuks, Aerosol Meckahincs (Publishing House of the Academy of Sciences of USSR, 1955) [in Russian].
S. Bohlmann, X. Shang, E. Giannakaki, M. Filioglou, S. Romakkaniemi, M. Komppula, and A. Saarto, “Action and characterization of birch pollen in the atmosphere using a multiwavelength Raman polarization lidar and hirst-type pollen sampler in Finland,” Atmos. Chem. Phys. 19 (23), 14559–14569 (2019).
P. J. Beggs, J. M. Davies, A. Milic, S. G. Haberl, F. H. Johnston, P. J. Jones, C. H. Katelaris, and E. Newbigin, Australian Airborne Pollen and Spore Monitoring Network Interim Standard and Protocols (Macquare University, Queensland University of Technology, 2018).
F. Tummon, L. A. Arboledas, M. Bonini, B. Guinot, M. Hicke, J. Christophe, V. Kendrovski, W. McCairns, E. Petermann, V. H. Peuch, O. Pfaar, M. Sicard, B. Sikoparija, and B. Clot, “The need for Pan-European automatic pollen and fungal spore monitoring: A stakeholder workshop position paper,” Clin. Transl. Allergy 11 (3), e12015 (2021). https://doi.org/10.1002/clt2.12015
G. S. Raynor, E. C. Ogden, and J. V. Haes, “Dispersion and deposition of ragweed pollen from experimental sources,” J. Appl. Meteorol. 9 (6), 885–895 (1970).
S. Blackmore and Y. S. Barnes, “Harmomegathic mechanisms in pollen grains,” in Pollen and Spores. Form and Function (Academic Press, London, 1986).
J. B. Harrington and M. Kurt, “Ragweed pollen density,” Am. J. Bot. 50 (6), 532–539 (1963).
J. Lacey, “Aggregation of spores and its effect on aerodynamic behavior,” Grana, No. 30, 437–445 (1991).
V. V. Golovko, K. P. Kutsenogii, and V. L. Istomin, “Agglomerate composition of pollen aerosol in the atmosphere of Novosibirsk,” Opt. Atmos. Okeana 27 (6), 553–559 (2014).
V. V. Golovko, A. P. Belanova, and G. A. Zueva, “Study of the cluster composition of pollen particles entering the atmosphere during the bloom of anemophilic plants,” Opt. Atmos. Okeana 32 (6), 476–481 (2019). https://doi.org/10.15372/AOO20190610
V. V. Golovko, G. A. Zueva, and T. I. Kiseleva, “Anemophilous plant pollen grains entering the atmosphere: Cluster composition,” Atmos. Ocean. Opt. 34 (5), 483–490 (2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Nikol’skii
Rights and permissions
About this article
Cite this article
Golovko, V.V., Zueva, G.A. & Kiseleva, T.I. Cluster Composition of Anemophilous Plant Pollen Entering the Atmosphere. Atmos Ocean Opt 35, 673–679 (2022). https://doi.org/10.1134/S1024856022060136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1024856022060136