Abstract
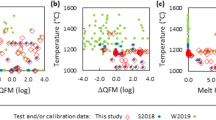
Using published experimental data an expression was derived for the Ti4+/Ti3+ ratio as a function of temperature, oxygen fugacity, and melt composition. The equation can be used to estimate Ti3+ content in lunar basaltic melts. It was shown that the Ti3+ content in melts is probably no higher than the Fe3+ content even under the reduced conditions typical of lunar magmas. Trivalent Ti can lead to some decrease in \(f_{O_2 }\) during melt cooling under closed-system conditions, but it cannot reduce Fe2+ in melt to metal, because it will be completely consumed by Fe3+ reduction to Fe2+. The presence of additional reducers, such as Cr2+, can be favorable for the formation of metal during melt cooling.
Similar content being viewed by others
References
Anderson, A.T., Bunch, T.E., Cameron, E.N., Haggerty, S.E., Boyd, F.R., Finger, L.W., James, O.B., Keil, K., Prinz, M., Ramdohr, P., and El Goresy, A., Armalcolite: a New Mineral from the Apollo 11 Samples, Proc. Apollo-11 Lunar Sci. Conf. Geochim Cosmochin Acta, 1970, vol. 34(Suppl. 1), pp. 55–63.
Ariskin, A.A., Borisov, A.A., and Petaev, M.I., Calculating Metal/Silicate Equilibria in Meteoritic Igneous Systems, Lunar Planet. Sci. Conf., 1996, vol. 27, pp. 37–38.
Ariskin, A.A., Petaev, M.I., Borisov, A.A., and Barmina, G.S., METEOMOD: a Numerical Model for the Calculation of Melting-Crystallization Relationships in Meteoritic Igneous Systems, Meteorit. Planet. Sci., 1997, vol. 32, pp. 123–133.
Bézos, A. and Humler, E., The Fe3+/ΣFe Ratio of MORB Glasses and Their Implications for Mantle Melting, Geochim. Cosmochim. Acta, 2005, vol. 69, pp. 711–725.
Berry, A.J. and O’Neill, H.St., A XANES Determination of the Oxidation State of Chromium in Silicate Glasses, Am. Mineral., 2004, vol. 89, pp. 790–798.
Berry, A.J., Shelley, M.G., Foran, G.J., O’Neill, H.St.C., and Scott, D.R., A Furnace Design for XANES Spectroscopy of Silicate Melts Under Controlled Oxygen Fugacities and Temperatures to 1773 K, J. Synchrotron Radiation, 2003, vol. 10, pp. 332–336.
Borisov, A. and McCammon, C., The Effect of Silica on Ferric/Ferrous Ratio in Silicate Melts: An Experimental Investigation Using Mössbauer Spectroscopy, Am. Mineral., 2010, vol. 95, pp. 545–555.
Borisov, A. and Palme, H., Experimental Determination of the Solubility of Pt in Silicate Melts, Geochim. Cosmochim. Acta, 1997, vol. 61, pp. 4349–4357.
Borisov, A. and Palme, H., Solubilities of Noble Metals in Iron-Containing Silicate Melts As Derived from Experiments in Iron-Free Systems, Am. Mineral., 2000, vol. 85, pp. 1665–1673.
Borisov, A., Brenker, F., and Palme, H., Liquidus Karrooite Stability and Composition at Reducing Conditions, Contrib. Mineral. Petrol., 2004, vol. 148, pp. 69–78.
Borisov, A., Lahaye, Y., and Palme, H., The Effect of Sodium on the Solubilities of Metals in Silicate Melts, Am. Mineral., 2006, vol. 91, pp. 762–771.
Borisov, A.A. and Shapkin, A.I., Hew Empirical Equation of Dependence of Fe3+/Fe2+ Ratio in Natural Melts on their Composition, Oxygen Fugacity, and Temperature, Geokhimiya, 1989, no. 6, pp. 892–897.
Borisov, A.A., Ferric-Ferrous Ratio in Liquid Iron Oxides: Analysis and Applications to Natural Basaltic Melts, Petrology, 2010, vol. 18, no. 5, pp. 471–481.
Borisov, A.A., Temperature Dependence of Redox Reaction with Participation of Elements of Variable Valence in the Model and Natural Melts, Geokhimiya, 1988, no. 5, pp. 706–714.
Brückner, R., Redox Behavior in Oxide Melts and Glasses, in Current topics on non-crystalline solids, Baro, M.D. and Clavaguera, N., Eds. Singapore: World Scientific Publ., 1986, pp. 109–140.
Brett, R., Butler, Jr.P., Meyer, Jr.C., Reid, A.M., Takeda, H., and Williams, R., Apollo-12 Igneous Rocks 12004, 12008, 120099 and 12022: a Mineralogical and Petrological Study, Proceedings of the Second Lunar and Planetary Science Conference. Geochim. Cosmochim. Acta, 1971, no. 2 (Suppl.), pp. 301–317.
Carmichael, I.S.E. and Ghiorso, M.S., Oxidation-Reduction Relations in Basic Magma: a Case for Homogeneous Equilibria, Earth Planet. Sci. Lett., 1986, vol. 78, pp. 200–210.
Cottrell, E. and Kelley, K.A., The Oxidation State of Fe in MORB Glasses and the Oxygen Fugacity of the Upper Mantle, Earth Planet. Sci. Lett., 2011, vol. 305, pp. 270–282.
Delano, J.W., Pristine Lunar Glasses-Criteria, Data, and Implications, J. Geophys. Res., 1986, vol. 91, pp. D201–D213.
French, B.M. and Eugster, H.P., Experimental Control of Oxygen Fugacities by Graphite-Gas Equilibriums, J. Geophys. Res., 1965, vol. 70, pp. 1529–1539.
Gaskell, D.R., Introduction to Thermodynamics of Materials, 3-rd ed, Washington, DC: Taylor and Francis, 1995.
Haslbeck, S., Martinek, K.-P., Stievano, L., and Wagner, F.E., Formation of Gold Nanoparticles in Gold Ruby Glass: The Influence of Tin, Hyperfine Interact., 2005, vol. 165, pp. 89–94.
Huebner, J.S. and Sato, M., The Oxygen Fugacity-Temperature Relationships of Manganese Oxide and Nickel Oxide Buffers, Am. Mineral., 1970, vol. 55, pp. 934–952.
Jayasuriya, K.D., O’Neil, H.St.C., Berry, A., and Campbell, S.J., A Mössbauer Study of the Oxidation State of Fe in Silicate Melts, Am. Mineral., 2004, vol. 89, pp. 1597–1609.
Johnston, W.D., Oxidation-Reduction Equilibria in Molten Na2O · 2SiO2 Glass, J. Am. Ceram. Soc., 1965, vol. 48, pp. 184–190.
Jones, J.H. and Palme, H., Geochemical Constraints on the Origin of the Earth and Moon, in Origin of the Earth and Moon, Canup, R.M. and Righter, K. Eds., Tucson: Univ. Arizona Press, 2000, pp. 197–216.
Kennedy, G.C., Equilibrium between Volatiles and Iron Oxides in Igneous Rocks, Am. J. Sci., 1948, vol. 246, pp. 529–549.
Krawczynski, M.J., Sutton, S.R., Barr, J.A., and Grove, T.L., Titanium Valence in Lunar Ultramafic Glasses and Olivine Diogenites, Lunar Planet. Sci. Conf., 2010, vol. 41, Abstr. no. 1825.
Morizane, Y., Ozturk, B., and Fruehan, R.J., Thermodynamics of TiOx in Blast Furnace-Type Slags, Metal. Mater. Trans., 1999, vol. 30B, pp. 29–43.
Myers, J. and Eugster, H.P., The System Fe-Si-O: Oxygen Buffer Calibrations to 1500 K, Contrib. Mineral. Petrol., 1983, vol. 82, pp. 75–90.
Nikolaev, G.S., Borisov, A.A., and Ariskin, A.A., Calculation of the Ferric-Ferrous Ratio in Magmatic Melts: Testing and Additional Calibration of Empirical Equations for Various Magmatic Series, Geochem. Int., 1996, vol. 34, no. 8, pp. 641–649.
Ottonello, G., Moretti, R., Marini, L., and Zuccolini, M.V., Oxidation State of Iron in Silicate Glasses and Melts: a Thermochemical Model, Chem. Geol., 2001, vol. 174, pp. 157–179.
Pyare, R. and Nath, P., Free Oxygen Ion Activity in Binary Alkali Silicate Glasses, J. Non-Cryst. Solids, 1991, vol. 128, pp. 154–161.
Reid, A.M. and Meyer, Jr., C., Harmon, R.S., and Brett, R., Metal Grains in Apollo-12 Igneous Rocks, Earth Planet. Sci. Lett., 1970, vol. 9, pp. 1–5.
Robie, R.A. and Hemingway, B.S., Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures, U.S. Geol. Surv. Bull., 1995, no. 2131.
Sack, R.O., Carmichael, I.S.E., Rivers, M., and Ghiorso, N.S., Ferric-Ferrous Equilibria in Natural Silicate Liquids at 1 Bar, Contrib. Mineral. Petrol., 1980, vol. 75, pp. 369–376.
Sato, M., Hickling, N.L., and McLane, J.E., Oxygen Fugacity Values of Apollo-12, -14, and -15 Lunar Samples and Reduced State of Lunar Magmas, Proc. 4th Lunar Science Conference, Geochim. Cosmochim. Acta, 1973, vol. 1, no. Suppl. 4, pp. 1061–1079.
Schreiber, H.D., Balazs, G.B., Shaffer, A.P., and Jamison, P.L., Iron Metal Production in Silicate Melts Through the Direct Reduction of Fe (II) by Ti (III), Cr (II), and Eu (II), Geochim. Cosmochim. Acta, 1982, vol. 46, pp. 1891–1901.
Schreiber, H.D., Thanyasiri, T., Lach, J.J., and Legere, R.A., Redox Equilibria of Ti, Cr, and Eu in Silicate Melts: Reduction Potentials and Mutual Interactions, Phys. Chem. Glasses, 1978, vol. 19, pp. 126–139.
Simon, S.B., Sutton, S.R., and Grossman, L., Valence of Titanium and Vanadium in Pyroxene in Refractory Inclusion Interiors and Rims, Geochim. Cosmochim. Acta, 2007, vol. 71, pp. 3098–3118.
Tranell, G., Ostrovski, O., and Jahanshahi, S., Silica Activity and Raman Spectra of the CaO-SiO2-TiOx System, High Temp. Mater. Processes, 2001, vol. 20, pp. 65–78.
Tranell, G., Ostrovski, O., and Jahanshahi, S., The Equilibrium Partitioning of Titanium between Ti3+ and Ti4+ Valency States in CaO-SiO2-TiOx Slags, Metal. Mater. Trans., 2002, vol. 33, pp. 61–67.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Borisov, 2012, published in Petrologiya, 2012, Vol. 20, No. 4, pp. 429–436.
Rights and permissions
About this article
Cite this article
Borisov, A.A. The Ti4+/Ti3+ ratio of magmatic melts: Application to the problem of the reduction of lunar basalts. Petrology 20, 391–398 (2012). https://doi.org/10.1134/S0869591112040030
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591112040030