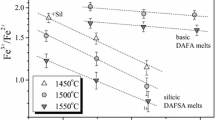
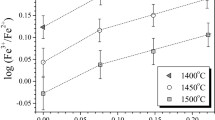
Abstract
Experimental data on the proportions of ferrous and ferric iron in pure liquid oxides (Darken and Gurry, 1946) were used to test different redox models. The obtained inferences were used to evaluate possible problems in describing the dependence of Fe3+/Fe2+ on oxygen fugacity in natural basaltic melts.
Similar content being viewed by others
References
A. A. Borisov and A. I. Shapkin, “New Empirical Equation of Dependence of Fe3+/Fe2+ Ratio in Natural Melts on their Composition, Oxygen Fugacity, and Temperature,” Geokhimiya, No. 6, 892–897 (1989).
A. Borisov and C. McCammon, “The Effect of Silica on Ferric/Ferrous Ratio in Silicate Melts: An Experimental Investigation using Mössbauer Spectroscopy,” Am. Mineral. 95(4), 545–555 (2010).
A. A. Borisov, “Temperature Dependence of Redox Reactions with Participation of Elements of Variable Valence in the Model and Natural Melts,” Geokhimiya, No. 5, 706–714 (1988).
A. Borisov, H. Palme, and B. Spettel, “Solubility of Pd in Silicate Melts: Implications for Core Formation in the Earth,” Geochim. Cosmochim. Acta 58, 705–716 (1994).
D. M. Christie, I. S. E. Carmichael, and C. H. Langmuir, “Oxidation State of Mid-Ocean Ridge Basalt Glasses,” Earth Planet. Sci. Lett. 79, 397–411 (1986).
L. S. Darken and R. W. Gurry, “The System Iron-Oxygen. I. The Wüstite Field and Related Equilibria,” J. Am. Chem. Soc. 67, 1398–1412 (1945).
L. S. Darken and R. W. Gurry, “The System Iron-Oxygen. II. Equilibrium and Thermodynamics of Liquid Oxide and Other Phases,” J. Am. Chem. Soc. 68, 798–816 (1946).
P. S. Deines, R. H. Nafziger, G. C. Ulmer, and E. Woermann, “Temperature-Oxygen Fugacity Tables for Selected Gas Mixtures in the System C-H-O at One Atmosphere Total Pressure,” Bull. Earth Miner. Sci., Exp. St., Pennsylv. St. Univ. No. 88 (1974).
R. F. Fudali, “Oxygen Fugacities of Basaltic and Andesitic Magmas,” Geochim. Cosmochim. Acta 29, 1063–1075 (1965).
K. D. Jayasuriya, H. St. C. O’Neil, A. Berry, and S. J. Campbell, “A Mössbauer Study of the Oxidation State of Fe in Silicate Melts,” Am. Mineral. 89, 1597–1609 (2004).
W. D. Johnston, “Oxidation-Reduction Equilibria in Iron-Containing Glass,” J. Am. Ceram. Soc. 47, 198–201 (1964).
G. C. Kennedy, “Equilibrium between Volatiles and Iron Oxides in Igneous Rocks,” Am. J. Sci. 246, 529–549 (1948).
A. Kilinc, I. S. E. Carmichael, M. L. Rivers, and R. O. Sack, “The Ferric-Ferrous Ratio of Natural Silicate Liquids Equilibrated in Air,” Contrib. Mineral. Petrol. 83, 136–140 (1983).
V. C. Kress and I. S. E. Carmichael, “Stoichiometry of the Iron Oxidation Reaction in Silicate Melts,” Am. Mineral. 73, 1267–1274 (1988).
V. C. Kress and I. S. E. Carmichael, “The Compressibility of Silicate Liquids Containing Fe2O3 and the Effect of Composition, Temperature, Oxygen Fugacity and Pressure on Their Redox States,” Contrib. Mineral. Petrol. 108, 82–92 (1991).
H. St. C. O’Neill and M. I. Pownceby, “Thermodynamic Data from Redox Reactions at High Temperatures. I. An Experimental and Theoretical Assessment of the Electrochemical Method Using Stabilized Zirconia Electrolytes, with Revised Values for the Fe-“FeO”, Co-CoO, Ni-NiO and Cu-Cu2O Oxygen Buffers, and New Data for the W-WO2 Buffer,” Contrib. Mineral. Petrol. 114, 296–314 (1993).
H. St. C. O’Neill, “Quartz-Fayalite-Iron and Quartz-Fayalite-Magnetite Equilibria and the Free Energy of Formation of Fayalite (Fe2SiO4) and Magnetite (Fe3O4),” Am. Mineral. 72, 67–75 (1987).
R. O. Sack, I. S. E. Carmichael, M. Rivers, and N. S. Ghiorso, “Ferric-Ferrous Equilibria in Natural Silicate Liquids at 1 Bar,” Contrib. Mineral. Petrol. 75, 369–376 (1980).
K. Shibata, “The Oxygen Partial Pressure of the Magma from Mihara Volcano, O-Sima, Japan,” Bull. Chem. Soc. Japan 40, 830–834 (1967).
C. R. Thornber, P. L. Roeder, and J. R. Foster, “The Effect of Composition on the Ferric-Ferrous Ratio in Basaltic Liquids at Atmospheric Pressure,” Geochim. Cosmochim. Acta 44, 525–532 (1980).
J. White, “Equilibrium at High Temperatures in Systems Containing Iron Oxides,” Carnegie Scholarship Mem. 27, 1–75 (1938).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Borisov, 2010, published in Petrologiya, 2010, Vol. 18, No. 5, pp. 494–504.
Rights and permissions
About this article
Cite this article
Borisov, A.A. Ferric-ferrous ratio in liquid iron oxides: Analysis and applications to natural basaltic melts. Petrology 18, 471–481 (2010). https://doi.org/10.1134/S0869591110050024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591110050024