Abstract
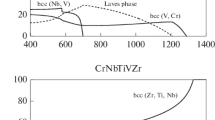
High-entropy alloys (HEAs) are the subject of attention of many scientific researchers. More than 200 thousand publications are known for HEAs and their number is growing every day. Particular interest in HEAs is caused by the peculiarities of their formation and structure, as well as the properties that such alloys have. In the material science classification, HEAs make up a special group, since the processes of structure and phase formation in them, the diffusion mobility of atoms, the mechanism of formation of mechanical properties, and thermal stability are significantly different from analogous processes in traditional alloys. The main feature of HEAs is the formation of a single-phase thermodynamically stable substitutional solid solution, mainly with an fcc or bcc lattice. To predict the formation of the structure of HEAs is a challenging problem to obtain information about new systems without using a complex and expensive experiment. Currently, there are two basic approaches to predicting the possible phase composition of HEAs in the world. The first approach implies the use of phenomenological parameters based on the Hume-Rothery criteria and thermodynamic parameters, and the second, the use of thermodynamic simulation. In this work, the probability of existence of the Al–Nb–Ti–V–Zr system as a HEA is considered for the following compositions: AlNbTiVZr0.25, AlNbTiVZr0.5, AlNbTiVZr, and AlNbTiVZr1.25. The phenomenological parameters are calculated, and the boundary conditions determining the stability and type of phases are revealed. CALPHAD is used to construct binary phase diagrams, which point to the possibility of formation of single-phase structures and intermetallic compounds during the formation of the alloy compositions under study. The results obtained demonstrate that the AlNbTiVZr-based are most likely to be disordered single-phase bcc solid solutions.


Similar content being viewed by others
REFERENCES
X. Yang, Y. Zhang, and B. Liaw, “Microstructure and compression properties of high-entropy alloys NbTiVTaAlx,” Procedia Eng. 36, 292–298 (2012).
N. Yu. Yurchenko, N. D. Stepanov, S. V. Zherebtsov, M. A. Tikhonovsky, and G. A. Salishchev, “Structure and mechanical properties of B2 ordered refractory AlNbTiVZrx (x = 0–1.5) high-entropy alloys,” Mater. Sci. Eng., A 704, 82–90 (2017).
B. Vishwanadh, N. Sarkar, S. Gangil, S. Singh, R. Tewari, G. K. Dey, and S. Banerjee, “Synthesis and microstructural characterization of a novel multicomponent equiatomic ZrNbAlTiV high entropy alloy,” Scr. Mater. 124, 146–150 (2016).
X. Sun, H. Zhang, S. Lu, X. Ding, Y. Wang, and L. Vitos, “Phase selection rule for Al-doped CrMnFeCoNi high-entropy alloys from first-principles,” Acta Mater. 140, 366–374 (2017).
Y. Zhang, Z. P. Lu, S. G. Ma, P. K. Liaw, Z. Tang, Y. Q. Cheng, and M. C. Gao, MRS Commun. 4.57 (2014).
M. C. Gao, J.-W. Yeh, P. K. Liaw, and Y. Zhang, High-Entropy Alloys. Fundamentals and Applications. https://doi.org/10.1007/978-3-319-27013-5
X. Yang and Zh. Yong, Mater. Chem. Phys. 132, 233–238 (2012). https://doi.org/10.1016/j.matchemphys.2011.11.021
S. Guo, C. Ng, J. Lu, and C. T. Lu, J. Appl. Phys. 109, 103505 (2011). https://doi.org/10.1063/L3587228
S. A. Krasikov, S. N. Agafonov, V. P. Chentsov, and E. M. Zhilina, “Influence of phase formation on the interphase interactions during the aluminothermic reduction of zirconium from its dioxide,” Russ. Metall. (Metally), No. 8, 615–618 (2015).
T. V. Osinkina, S. A. Krasikov, E. M. Zhilina, S. N. Agafonov, L. B. Vedmid’, and S. V. Zhidovinova, “Influence of niobium and tantalum on the phase formation during the metallothermic interaction of aluminum with titanium dioxide,” Russ. Metall. (Metally), No. 2, 85–89 (2019).
A. Dębski, R. Dębski, and W. Gasior, “New features of Entall database: comparison of experimental and model formation enthalpies,” Arch. Metall. Mater. 59 (4), 1337–1343 (2014).
M. C. Gao and D. E. Alman, “Searching for next single-phase high-entropy alloy compositions,” Entropy 15, 4504–4519 (2013).
The Periodic Table of the Elements by WebElements. http://www.webelements.com/.
Boiling and Melting Temperatures of Metals. Melting Temperature of Steel. http://thermalinfo.ru/svojstva-materialov/metally-i-splavy/temperatura-plavleniya-i-kipeniya-metallov-plotnost-i- teploprovodnost.
L. N. Mishenina and V. V. Shelkovnikov, Atomic Radii. http://dpva.ru/Guide/GuidePhysics/Length/Atomic-Radius/.
Funding
This work was carried out within the framework of a state assignment to the Institute of Metallurgy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Mityushova, Y.A., Gibadullina, A.F., Zhilina, E.M. et al. Thermodynamic Estimation of the Formation of a High-Entropy Al–Nb–Ti–V–Zr Alloy. Russ. Metall. 2021, 187–191 (2021). https://doi.org/10.1134/S0036029521020166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521020166