Abstract
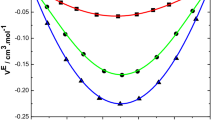
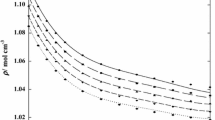
Density (ρ), viscosity (η), and ultrasonic velocity (U) are studied for binary liquid mixtures of chlorpheniramine with 1-butanol, 1-pentanol, and 1-hexanol at 303 K. Adiabatic compressibility (β), free length (Lf), free volume (Vf), internal pressure (πi), viscous relaxation time (τ), and Gibbs free energy (ΔG) are calculated by using the experimental data. The excess values of the above parameters (βE, \(L_{{\text{f}}}^{{\text{E}}}\), \(V_{{\text{F}}}^{{\text{E}}}\), \(\pi _{{\text{i}}}^{{\text{E}}}\), τE, and ΔGE) from their ideal values are also determined and fitted with Redlich–Kister polynomial equation. The changes in the determined parameters are interpreted in terms of the intermolecular interaction between the liquid mixtures. The significant changes in the ideal parameters and the excess parameters have confirmed the existence of the intermolecular interactions between the selected liquid system. From the observations, strength of intermolecular interaction of chlorpheniramine with the selected alcohols is in the order of 1‑butanol < 1-pentanol < 1-hexanol.



Similar content being viewed by others
REFERENCES
H. Agarwal, L. Kumari, L. Sinha, et al., Braz. J. Phys. 51, 515 (2021).
P. J Darolia, S. Malik, S. Garg, et al., J. Solution Chem. 50, 355 (2021).
L. Palaniappan and S. Nithiyanantham, Chem. Africa. 3, 277 (2020).
A. Awasthi, V. Verma, R. K. Tiwari, et al., J. Math. Chem. 58, 2291 (2020).
S. Verma, S. Gahlyan, M. Rani, et al., J. Mol. Liq. 274, 300 (2019).
B. Srikanth, M. Gowrisankar, S. Babu, et al., Russ. J. Phys. Chem. A 94, 2544 (2020).
A. Shakila, S. Ravikumar, V. Pandiyan, et al., J. Mol. Liq. 285, 279 (2019).
P. Nagababu, S. Babu, D. F. Santos, et al., Phys. Chem. Liq. 57, 689 (2019).
B. Satheesh, D. Sreenu, T. Savitha Jyostna, et al., Chem. Data Collect. 28, 100448 (2020).
K. Dharmalingam, K. Ramachandran, and P. Sivagurunathan, Phys. B (Amsterdam, Neth.) 139, 127 (2007).
S. Thirumaran and E. Jayakumar, Indian J. Pure Appl. Phys. 47, 265 (2009).
O. Redlich and A. T. Kister, Ind. Eng. Chem. 40, 345 (1948).
T. Chen, X. Feng, Y. Yin, et al., J. Solution Chem. 49, 1402 (2020).
J. P. Bazile, D. Nasri, H. Hoang, et al., Int. J. Thermophys. 41, 115 (2020).
E. Sampandam, T. Diriba Garbi, and Y. Alemu Abbo, Chem. Africa. 3, 1101 (2020).
S. Elangovan and S. Mullainathan, Russ. J. Phys. Chem. A 90, 1006 (2016).
B. Jacobson, J. Chem. Phys. 20, 927 (1952).
R. Reimann and A. Heintz, J. Solution Chem. 20, 29 (1991).
R. J. Fort and W. R. Moore, Trans. Faraday Soc. 62, 1112 (1966).
S. Elangovan and S. Mullainathan, Russ. J. Phys. Chem. A 88, 2108 (2014).
R. Rajalakshmi, S. Ravikumar, K. Sivakumar, et al., Chem. Data Collect. 24, 100299 (2019).
G. P. Dubey and L. Dhingra, J. Mol. Liq. 318, 114072 (2020).
Funding
The work is supported by the Research and Technology Transfer Centre, Wollega University, Nekemte, Ethiopia. Project code WU/S1/108.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Elangovan, S., Kebede, L. & Senbeto, E.K. Intermolecular Interactions between Chlorpheniramine with 1-Butanol, 1-Pentanol, and 1-Hexanol. Russ. J. Phys. Chem. 96 (Suppl 1), S1–S7 (2022). https://doi.org/10.1134/S0036024422140084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422140084