Abstract
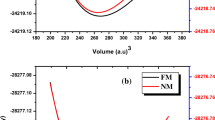
The structural, thermodynamic, and elastic properties of the cubic type B1 (NaCl) phase for the lead chalcogenides PbS, PbSe, and PbTe were studied using DFT calculations. The exchange-correlation functional used in this study is the generalized gradient approximation associate by Perdew–Burke–Ernzerhof (GGA-PBE). The structural properties of these compounds have been calculated and their values have been found in good agreement with the experimental results. The stability of these materials is discussed on the basis of the calculated elastic constants Cij, in which the results indicate that the studied compounds are stable and the estimated values are in excellent agreement with the experimental data. The thermodynamic properties of PbX materials have also been evaluated. The influence of temperature and pressure upon the bulk modulus (B), expansion coefficients (α), and heat capacities Cv and Cp were estimated and discussed. The heat capacity Cv reaches up to 49 J/mol K for different materials studied. The influence of temperature on the Cp was also investigated for different pressures (from 0 to 12 GPa) and the results obtained were analyzed.




Similar content being viewed by others
REFERENCES
D. Khokhlov, Lead Chalcogenides: Physics and Applications (Boca Raton: CRC Press, 2002).
S. V. Ovsyannikov, V. V. Shchennikov, S. V. Popova, and A. Y. Derevskov, Phys. Status Solidi B 235, 521 (2003). https://doi.org/10.1002/pssb.200301614
Y. Pei, A. LaLonde, S. Iwanaga, and G. J. Snyder, Energy Environ. Sci. 4, 2085 (2011). https://doi.org/10.1039/c0ee00456a
S. Wang, J. Zhang, Y. Zhang, et al., Inorg. Chem. 52, 8638 (2013). https://doi.org/10.1021/ic400801s
R. S. Allgaier, Phys. Rev. 112, 828(1958). https://doi.org/10.1103/PhysRev.112.828
R. S. Allgaier, J. Appl. Phys. 32, 2185(1961). https://doi.org/10.1063/1.1777039
J. R. Burke, B. Houston, and H.T. Savage, Phys. Rev. B 2, 1977 (1970). https://doi.org/10.1103/PhysRevB.2.1977
K. Hummer, A. Gruneis, and G. Kresse, Phys. Rev. B 75, 195211 (2007). https://doi.org/10.1103/PhysRevB.75.195211
H. Preier, Appl. Phys. 20, 189 (1979). https://doi.org/10.1007/BF00886018
H. Zogg, A. Fach, C. Maissen, et al., Opt. Eng. 33, 1440 (1994). https://doi.org/10.1117/12.165808
M. L. Cohen and J. R. Chelikowsky, Electronic Structure and Optical Properties of Semiconductors, Springer Ser. Solid-State Sci., Vol. 75 (Springer, Berlin, Heidelberg, 1988).
S. H. Wei and A. Zunger, Phys. Rev. B 55, 13605 (1997). https://doi.org/10.1103/PhysRevB.55.13605
O. K. Andersen, Phys. Rev. B 12, 3060 (1975). https://doi.org/10.1103/PhysRevB.12.3060
D. Singh, H. Krakauer, and C. S. Wang, Phys. Rev. B 34, 8391 (1986). https://doi.org/10.1103/PhysRevB.34.8391
W. Kohn and L. J. Sham, Phys. Rev. B 140, A1133 (1965). https://doi.org/10.1103/PhysRev.140.A1133
R. Maizi, A.-G. Boudjahem, and M. Boulbazine, Russ. J. Phys. Chem. A 93, 2726 (2019). https://doi.org/10.1134/S0036024419130181
X. Gonze, J. M. Beuken, R. Caracas, et al., Comput. Mater. Sci. 25, 478 (2002). https://doi.org/10.1016/S0927-0256(02)00325-7
ABINIT. http://www.abinit.org.
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
A. S. Zyubin, T. S. Zyubina, Yu. A. Dobrovol’skii, et al., Russ. J. Inorg. Chem. 58, 1489 (2013). https://doi.org/10.1134/S0036023613120255
T. S. Zyubina and T. S. Dzhabiev, Russ. J. Inorg. Chem. 63, 1461 (2018). https://doi.org/10.1134/S0036023618110220
M. Lach-hab, D. A. Papaconstantopoulos, and M. J. Mehl, J. Phys. Chem. Solids. 63, 833 (2002). https://doi.org/10.1016/S0022-3697(01)00237-2
H. J. Monkhorst and J. D. Park, Phys. Rev. B 13, 5188 (1976). https://doi.org/10.1103/PhysRevB.13.5188
J. R. Macdonald and D. R. Powell, J. Res. Natl. Bur. Stand. A 75, 441 (1971).
F. D. Murnaghan, Proc. Natl. Acad. Sci. USA 30, 244 (1947). https://doi.org/10.1073/pnas.30.9.244
O. Madelung, M. Shulz, H. Weiss (Eds.), Numerical Data and Functional Relationships in Science and Technology, Landolt-Bornstei, New Series, Vol. 17 (Springer, Berlin, 1983).
O. Madelung, U. Rossler, M. Schulz (Eds.), Semiconductors: Group IV Elements, IV–IV and III–IV Compounds, Landolt-Bornstein, New series, Group III, Vol. 41, Pt. A (Springer-Verlag, Berlin, 2005).
Y. Bencherif, A. Boukra, A. Zaoui, and M. Ferhat, Mater. Chem. Phys. 126, 707 (2011). https://doi.org/10.1016/j.matchemphys.2010.12.056
Z. Wu, R. E. Cohen, Phys. Rev. B 73, 235116 (2006). https://doi.org/10.1103/PhysRevB.73.235116
M. Lach-hab, D. A. Papaconstantopoulos, and M. J. Mehl, J. Phys. Chem. Solids. 63, 833 (2002). https://doi.org/10.1016/S0022-3697(01)00237-2
M. J. Mehl, J. E. Osburn, D. A. Papaconstantopoulos, and B. M. Klein, Phys. Rev. B 41, 10311 (1990). https://doi.org/10.1103/physrevb.41.10311
M. J. Mehl, Phys. Rev B 47, 2493 (1993). https://doi.org/10.1103/PhysRevB.47.2493
Funding
This study was supported by the Algerian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Maizi, R., Ksouri, R., Boudjahem, AG. et al. First-Principles Calculations of Structural, Thermodynamic, and Elastic Properties of Lead Chalcogenides PbX (X = S, Se, and Te) in NaCl (B1) Phase. Russ. J. Inorg. Chem. 66, 2084–2090 (2021). https://doi.org/10.1134/S0036023621140023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621140023