Abstract
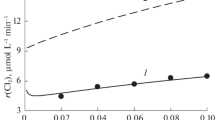
The kinetics of mineralization of oxalic acid Н2С2О4 under the action of ozone in acidic aqueous solution (С(НClО4) = 0.1 M, pH ~ 1) with the addition of \({\text{MnO}}_{4}^{ - }\) or Mn2+ ions was studied. It was found that manganese ions are effective catalysts for the reaction of O3 with oxalic acid. Regardless of the manganese species (\({\text{MnO}}_{4}^{ - }\) or Mn2+) added to the solution, it was converted into an oxalate complex of tetravalent manganese in the course of the reaction, and this complex was the stable form of the catalyst in the system under consideration. The kinetics of release of carbon dioxide—a product of the reaction of Н2С2О4 with О3—was determined depending on the concentrations of ozone in the gas stream and oxalic acid and manganese in the solution. A basic scheme of the catalysis of the test reaction by manganese ions was proposed, and a kinetic model was constructed to adequately describe the experimental results. The reaction scheme is based on the fact that oxalate is oxidized to CO2 in the course of a complex decomposition reaction of the oxalate complex of tetravalent manganese; in this case, Mn(IV) is reduced to Mn2+. The complex is regenerated by the oxidation of Mn2+ to Mn(IV) with ozone.







Similar content being viewed by others
REFERENCES
Marcì, G., García–López, E., and Palmisano, L., J. Appl. Electrochem., 2008, vol. 38, no. 7, p. 1029.
Bangun, J. and Adesina, A.A., Appl. Catal., A, 1998, vol. 175, no. 1, p. 221.
Von Sonntag, C. and Von Gunten, U., Chemistry of Ozone in Water and Wastewater Treatment. From Basic Principles to Applications, London: IWA, 2012.
Michael, K.M., Rizvi, G.H., Mathur, J.N., and Ramanujam, A., J. Radioanal. Nucl. Chem., 2000, vol. 246, no. 2, p. 355.
Ganesh, S., Desigan, N., Chinnusamy, A., and Pandey, N.K., J. Radioanal. Nucl. Chem., 2021, vol. 328, no. 3, p. 857.
Anan’ev, A.V., Tananaev, I.G., and Shilov, V.P., Russ. Chem. Rev., 2005, vol. 74, no. 11, p. 1039.
Seliverstov, A.F., Lagunova, Yu.O., Ershov, B.G., and Shashkovskii, S.G., Russ. J. Gen. Chem., 2017, vol. 87, no. 11, p. 2533.
Hoigné, J. and Bader, H., Water Res., 1983, vol. 17, no. 2, p. 185.
Gogolev, A.V., Shilov, V.P., Garnov, A.Yu, and Anan’ev, A.V., Radiochemistry, 2006, vol. 48, no. 1, p. 31.
Andreozzi, R., Insola, A., Caprio, V., and D’amore, M.G., Water Res., 1992, vol. 26, no. 7, p. 917.
Andreozzi, R., Caprio, V., D’amore, M.G., and Insola, A., Environ. Technol., 1995, vol. 16, no. 9, p. 885.
Andreozzi, R., Caprio, V., Insola, A., Marotta, R., and Tufano, V., Water Res., 1998, vol. 32, no. 5, p. 1492.
Ma, J. and Graham, N.J.D., Water Res., 2000, vol. 34, no. 15, p. 3822.
Qin, W., Tan, P., Song, Y., Wang, Z., Nie, J., and Ma, J., Sep. Purif. Technol., 2021, vol. 261, p. 118272.
Xiao, H., Liu, R., Zhao, X., and Qu, J., J. Mol. Catal. A: Chem., 2008, vol. 286, no. 1, p. 149.
Dong, Y., Yang, H., He, K., Song, S., and Zhang, A., Appl. Catal., B, 2009, vol. 85, no. 3, p. 155.
Reisz, E., Leitzke, A., Jarocki, A., Irmscher, R., and Von Sonntag, C., J. Water Supply: Res. Technol.–AQUA, 2008, vol. 57, no. 6, p. 451.
Levanov, A.V., Kuskov, I.V., Antipenko, E.E., and Lunin, V.V., Russ. J. Phys. Chem., 2006, vol. 80, no. 4, p. 556.
Levanov, A.V., Isaikina, O.Y., and Kharlanov, A.N., Russ. J. Phys. Chem. A, 2020, vol. 94, no. 11, p. 2219.
Levanov, A.V., Isaikina, O.Y., Gasanova, R.B., and Lunin, V.V., Russ. J. Phys. Chem. A, 2017, vol. 91, no. 8, p. 1427.
Levanov, A.V., Kuskov, I.V., Zosimov, A.V., Antipenko, E.E., and Lunin, V.V., J. Anal. Chem., 2003, vol. 58, no. 5, p. 439.
Yukichi, Y., Iwao, T., Masakazu, K., and Takashi, U., Bull. Chem. Soc. Jpn., 1974, vol. 47, no. 11, p. 2787.
Cartledge, G.H. and Ericks, W.P., J. Am. Chem. Soc., 1936, vol. 58, no. 10, p. 2069.
Takashi, U., Iwao, T., and Yukichi, Y., Bull. Chem. Soc. Jpn., 1975, vol. 48, no. 10, p. 2809.
Martell, A.E. and Smith, R.M., Critical Stability Constants, vol. 3. Other Organic Ligands, Boston: Springer, 1977.
Kettler, R.M., Palmer, D.A., and Wesolowski, D.J., J. Solution Chem., 1991, vol. 20, no. 9, p. 905.
Adler, S.J. and Noyes, R.M., J. Am. Chem. Soc., 1955, vol. 77, no. 8, p. 2036.
Tyupalo, N.F. and Yakobi, B.A., Zh. Neorg. Khim., 1980, vol. 25, no. 6, p. 1557.
Jacobsen, F., Holcman, J., and Sehested, K., Int. J. Chem. Kinet., 1998, vol. 30, no. 3, p. 207.
Levanov, A.V., Kuskov, I.V., Antipenko, E.E., and Lunin, V.V., Russ. J. Phys. Chem. A, 2008, vol. 82, no. 7, p. 1126.
Levanov, A.V., Isaikina, O.Y., and Lunin, V.V., Russ. J. Phys. Chem. A, 2020, vol. 94, no. 1, p. 81.
Funding
This work was carried out within the framework of the state assignment “Surface Physicochemistry, Adsorption, and Catalysis.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Levanov, A.V., Isaikina, O.Y. & Gryaznov, R.A. Catalytic Ozonation of Oxalic Acid in Aqueous Solution in the Presence of Manganese Ions. Kinet Catal 63, 180–187 (2022). https://doi.org/10.1134/S0023158422020069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422020069