Abstract
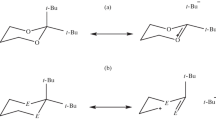
Computer simulation of pathways of conformational isomerization of 1,3-oxathiane molecule carried out with the help of HF/6-31G(d), MP2.6-31G(d)/HF/6-31G(d), and PNE/3z quantum-chemical approximations showed that interconversion between the degenerate in energy chair conformers proceeds through seven independent pathways: directly and via six flexible forms. Potential energy surface contains eight minimum points including chair conformers and enantiomeric pairs of twist forms, and also five transition states, among them different modification of semi-chair, symmetric and unmmetrical boat. Molecular dynamics methods show that flexible forms at room temperature convert into one another and into the chair conformers.
Similar content being viewed by others
References
Nakano, H., Takahashi, K., Suzuki, Y., and Fujita, R., Tetrahedron Asymmetry, 2005, vol. 16, no. 3, p. 609.
Okuyama, Y., Nakano, H., Saito, Y., Takahashi, K., and Hongo, H., Tetrahedron Asymmetry, 2005, vol. 16, no. 15, p. 2551.
Aggarwal, V.K., Lattanzi, A., and Fuentes, D., Chem. Commun., 2002, no. 21, p. 2534.
Matsubara, S., Kasuga, J., Yasui, T., Yoshida, M., Yamin, B., Utimoto, K., and Oshima, K., Chirality, 2003, vol. 15, no. 1, p. 38.
Nakano, H., Okuyama, Y., Yanagida, M., and Hongo, H., J. Org. Chem., 2001, vol. 66, no. 2, p. 620.
Rakhmankulov, D.L., Zorin, V.V., Latypova, F.N., Zlotskii, S.S., and Karakhanov, R.A., Usp. Khim., 1983, vol. 52, no. 4, p. 619.
Zefirov, N.S. and Kazimirchik, I.V., Usp. Khim., 1974, vol. 43, no. 2, p. 252.
Gren’, A.I., Kuznetsov, V.V., and Bacherikov, V.A., Ukr. Khim. Zh., 1999, vol. 65, no. 10, p. 73.
Gelan, J. and Anteunis, M., Bull. Soc. Chim. Belges, 1968, vol. 77, no. 3, p. 423.
Galan, J. and Antheunis, M., Bull. Soc. Chim. Belges, 1970, vol. 49, no. 2, p. 313.
Allingham, Y., Crabb, T.A., and Newton, R.F., Org. Magn. Res., 1971, vol. 3, no. 1, p. 37.
Pasanen, P., Suomen Kemistilehti, 1972, vol. 45, no. 5, p. 373.
Bogatskii, A.V., Turyanskaya, A.M., Gren’, A.I., Bal’trush, E., and Waigt, A., Vopr. Stereokhim., 1974, no. 4, p. 49.
Pihlaja, K., Pasanen, P., and Wahasilta, J., Org. Magn. Res., 1979, vol. 12, no. 5, p. 321.
Turyanskaya, A.M., Gorbatyuk, V.Ya., Kuznetsov, V.V., and Gren’, A.I., Zh. Obshch. Khim., 1992, vol. 62, no. 8, p. 1892.
Turyanskaya, A.M., Timofeev, O.S., Kuznetsov, V.V., and Gren’, A.I., Zh. Org. Khim., 1992, vol. 27, no. 1, p. 79.
Turyanskaya, A.M., Timofeev, O.S., Kuznetsov, V.V., and Gren’, A.I., Zh. Org. Kkim., 1995, vol. 21, no. 1, p. 132.
Kleinpeter, E., Koch, A., and Pihlaja, K., Tetrahedron, 2005, vol. 61, no. 31, p. 7349.
Stuparu, M., Grosi, I., Muntean, L., Ple, G., Cisnas, C., Teres, A., Nan A., and Moger, C., Monatcsh. Chem., 2004, vol. 135, no. 1, p. 89.
Alabugin, I.V., J. Org. Chem., 2000, vol. 65, no. 13, p. 3910.
Alabugin, I.V., Manoharan, M., and Zeidan, T.A., J. Am. Chem. Soc., 2003, vol. 125, no. 46, p. 14014.
Vnutrenneye vrashchenie molekul (Internal Rotation of Molecules), Orvill-Thomas, V.J., Ed., Moscow: Mir, 1975, p. 352.
Freeman, F. and Uyen Do, K., J. Mol. Struct. (THEOCHEM), 2002, vol. 577, p. 43.
Mazitova, E.G., Kuramshina, A.E., and Kuznetsov, V.V., Zh. Org. Khim., 2004, vol. 40, no. 4, p. 615.
Kuramshina, A.E., Bochkor, S.A., and Kuznetsov, V.V., Zh. Org. Khim., 2006, vol. 42, no. 4, p. 615.
Kuramshina, A.E. and Kuznetsov, V.V., Khim. Geterotsikl. Soed., 2009, no. 1, p. 131.
Kuramshina, A.E., Bochkor, S.A., and Kuznetsov, V.V., Zh. Org. Khim., 2009, vol. 45, no. 4, p. 511.
Kuramshina, A.E. and Kuznetsov, V.V., Zh. Org. Khim., 2010, vol. 46, no. 6, p. 875.
Faizullin, M.G., Kuramshina, A.E., Mamleev, A.Kh., and Kuznetsov, V.V., Zh. Obshch. Khim., 2009, vol. 79, no. 12, p. 2046.
Kuznetsov, V.V., Kuramshina, A.E., and Bochkor, S.A., Zh. Org. Khim., 2009, vol. 45, no. 8, p. 1265.
Kuramshina, A.E., Bochkor, S.A., and Kuznetsov, V.V., Khim. Geterotsikl. Soed., 2009, no. 5, p. 686.
Kuznetsov, V.V., Kuramshina, A.E., and Bochkor, S.A., Zh. Strukt. Khim., 2009, vol. 50, no. 5, p. 960.
Freeman, F. and Thuy Le, K., J. Phys. Chem., 2003, vol. 107, no. 16, p. 2908.
Kuznetsov, V.V., Zh. Org. Khim., 2010, vol. 46, no. 11, p. 1660.
Turyanskaya, A.M., Novikov, A.N., Verhivker, G.M., and Kuznetsov, V.V., Zh. Obshch. Khim., 2001, vol. 71, no. 9, p. 1571.
Turyanskaya, A.M. and Kuznetsov, V.V., Khim. Geterotsikl. Soed., 2002, no. 5, p. 692.
Kuznetsov, V.V., Zh. Org. Khim., 2010, vol. 46, no. 1, p. 117.
Kuznetsov, V.V., Zh. Org. Khim., 2010, vol. 46, no. 10, p. 1576.
Kuznetsov, V.V., Zh. Obshch. Khim., 2011, vol. 81, no. 1, p. 151.
Bochkor, S.A. and Kuznetsov, V.V., Zh. Org. Khim., 2010, vol. 46, no. 6, p. 943.
Kuznetsov, V.V., Khim. Geterotsikl. Soed., 2911, no. 1, p. 144.
HyperChem 7.10, Trialversion.www.hyper.com.
Laikov, D.N. and Ustynyuk, Yu.A., Izv. Ross. Akad. Nauk, Ser. Khim., 2005, no. 3, p. 304.
De Wolf, N., Verschoor, G.C., and Romers, C., Acta Cryst. C, 1972, vol. 28, no. 8, p. 2424.
Kuznetsov, V.V., Zh. Obshch. Khim., 2010, vol. 80, no. 12, p. 2030.
Valiakhmetova, O.Yu. and Kuznetsov, V.V., Zh. Org. Khim., 2010, vol. 46, no. 9, p. 1368.
Valiakhmetova, O.Yu. and Kuznetsov, V.V., Khim. Geterotsikl. Soed., 2010, no. 3, p. 460.
Friebolin, H., Schmid, H.G., Kabuss, S., and Faisst, W., Org. Magn. Res., 1969, vol. 1, no. 1, p. 67.
Eliel, E.L. and Juaristi, E., J. Am. Chem. Soc., 1978, vol. 100, no. 19, p. 6114.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Kuznetsov, 2012, published in Zhurnal Obshchei Khimii, 2012, Vol. 82, No. 5, pp. 764–769.
Rights and permissions
About this article
Cite this article
Kuznetsov, V.V. Conformational analysis of 1,3-oxathiane. Russ J Gen Chem 82, 869–873 (2012). https://doi.org/10.1134/S1070363212050118
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363212050118