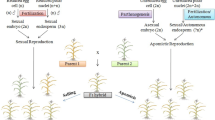
Summary
Little is known about the genetic basis and molecular mechanisms regulating female gametogenesis in flowering plants. In many species sexuality is replaced by apomixis, a method of asexual reproduction that circumvents female meiosis and fertilization, and culminates in the formation of clonal seeds. Using a new generation of transposon based insertional mutagenesis strategies and their resulting molecular tools, we are investigating how female meiotically derived cells (megaspores) acquire their identity. We are also determining their function and interactions, and attempting the induction of apomixis initiation in the ovule of Arabidopsis. This basic knowledge will contribute to establish the transfer of apomixis into sexual crops, a major challenge faced by plant biotechnology. The introduction of apomixis as a reproductive alternative could represent a unique opportunity to simplify breeding schemes and genetically perpetuate any desired heterozygous genotype, including hybrids.
Similar content being viewed by others
References
Asker, S. E.; Jerling, L. Apomixis in plants. Boca Raton, FL: CRC Press; 1992.
Beadle, G. W.; McClintock, B.. A genetic disturbance of meiosis in Zea mays. Science 68:433: 1928.
Bell, P. R. Incompatibility in flowering plants: adaptation of an ancient response. Plant Cell 7:5–16; 1995.
Bellen, H. J.; O'Kane, C. J.; Wilson, C.; Grossniklaus, U.; Pearson, R. K.; Gehring, W. J.; P-element mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 3:1288–1300; 1989.
Bicknell, R. A. Isolation of a diploid, apomictic plant of Hieracium aurantiacum. Sex. Plant Reprod. 10:168–172; 1997.
Bretagnolle, F.; Thompson, J. D. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 129:1–22; 1995.
Carman, J. G. Asynchronous expression of duplicate genes in angiosperms may cause apomixes, bispory, tetraspory and polyembryony. J. Linnean Soc. 61:51–94; 1997.
Carman, J. G.; Crane, C. F.; Rieralizarazu O. Comparative histology of cell walls during meiotic and apomeiotic megasporogenesis in two hexaploid Australasian Elymus species. Crop. Sci. 31(6):1527–1532; 1991.
Conteau, F.; Belzile, F.; Horlow, C.; Grandjean, O.; Vezon, D.; Doutriaux, M. P. Random chromosome segregation without meiotic arrest in both male and female meiocytes of a dmel mutant of Arabidopsis. Plant Cell 11(9):1623–1634; 1999.
D'Amato, F. Polyploidy in cell differentiation. Caryologis 42:183–211; 1989.
De Haan, A.; Maceira, N. O.; Lumaret, R.; Delay, J. Production of 2n gametes in diploid subspecies of Dactylis glomerata L. 2. Occurrence and frequency of 2n eggs. Ann. Bot. 69:345–350; 1992.
Golubovkaya, I. N. Effect of several meiotic mutants on female meiosis in maize. Dev. Genet. 13:411–424; 1992.
Golubovskaya, I. N.; Avalkin, N. A.; Sheridan, W. F. New insights into the role of the maize ameiotic 1 locus. Genetics 147:1339–1350; 1997.
Golubovskaya, I. N.; Grebennikova, Z. K.; Avalkina, N. A.; Sheridan, W. F. The role of the ameiotic 1 gene in the initiation of meiosis and in subsequent meiotic events in maize. Genetics 135:1115–1166; 1993.
Golubovskaya, I. N.; Sitnikova, D. V. Three meiotic mutations disturbing chromosome segregation at the first meiotic division in corn. Genetica 16:656–666; 1980.
Grimanelli D.; Leblane, O.; Espinosa, E.; Perotti, R.; De Leon D. G.; Savidan, Y. Mapping diplosporous apomixes in tetrapoid Tripsacum, one gene or several genes. Heredity 80:40–47; 1998.
Grossniklaus, U.; Bellen, H. J.; Wilson, C.; Gehring, W. J. P-element mediated enhancer detection applied to the study of oogenesis in Drosophila. Development 107:189–200; 1989.
Grossniklaus, U.; Pearson, R. K.; Gehring, W. J. The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev. 6:1030–1051; 1992.
Grossniklaus, U.; Schneitz, K. The molecular and genetic basis of ovule and megagametophyte development. Sem. Cell Dev. Biol. 9:227–238; 1998.
Gustafsson, Å. Apomixis in angiosperms II. Lunds Univ. Årsskr N F II 43:71–179; 1947.
Harlau, J. R.; de Wet, J. M. J. Pathways of genetic transfer from Tripsacum to Zea mays. Proc. Natl Acad. Sci. USA 74:3494–3497; 1977
Hermsen, J. G. T. Mechanisms and genetic implications of 2n-gamete formation Iowa State. J. Res. 58:421–434; 1984.
Jongedijk, E. Desynapsis and FDR 2N-megaspore formation in diploid potato: potentials and limitations for breeding and for the induction of diplosporic apomixis. PhD Dissertation, University of Wageningen; 1991:111 pp.
Kimber, G.; Riley, H. Haploid angiosperms. Bot. Rev. 29:480–531; 1963.
Knox, R. B. Apomixis: seasonal and population differences in a grass. Science 157:325–326; 1967.
Koltunow, A. M. Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5:1425–1437; 1993.
Koltunow, A. M.; Soltys, N.; Nobumasa, N.; McClure, S. Anther, ovule, and nucellar embryo development in Citrus sinensis cv Valencia. Can. J. Bot. 73:1567–1582; 1995.
Leblanc, O.; Mazzucato, A. Screening procedures to identify and quantify apomixis. In: Savidan, Y.; Carman, J. G.; Dresselhaus, T., eds., The flowering apomixis: from mechanisms to genetic engineering. Mexico, D.F.: CIMMYT, IRD, European Commission DG VI (FAIR); 2001:121–136.
Leblane, O.; Peel, M. D.; Carman, J. G.; Savidan, Y. Megasporogenesis and megagametogenesis in several Tripsacum species (Poaceae). Am. J. Bot. 82:57–63; 1995.
Ligrone, R.; Duckett, J. G.; Renzaglia, K. S. The gametophytic-sporophytic junction in land plants. Adv. Bot. Res. 19:231–317; 1993.
Lin, Y.-G.; Mitzukawa, N.; Oosumi, T.; Whittier, R. F. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8:457–463; 1995.
Maheswari, P. An introduction to the embryology of angiosperms. New York: McGraw-Hill Book Co., Inc.: 1950:453 pp.
Maizonnier, D. Production de tetraploïdes et de trisomiques naturels chez le Pétunia. Ann. d'Amel. Plantes 26:305–318; 1976.
Miller, O. L. Cytological studies of asynaptic maize. Genetics 48:1445–1466; 1963.
Naumova, T. N.; Willemse, M. T. M. Ultrastructural characterization of apospory in Panicum maximum. Sex. Plant Reprod. 8:197–204; 1995.
Nogler, G. A. Gametophytic apomixis. In: Johri, B. M., ed. Embryology of angiosperms. New York: Springer Verlag: 1984:475–518.
O'Kane, C. J.; Gehring, W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl Acad. Sci USA 85:9123–9127; 1987.
Parrott, W. A.; Smith, R. R. Production of 2n pollen in red clover. Crop Sci. 24:469–472; 1986.
Peel, M. D.; Carman, J. G.; Leblane, O. Megasporocyte callose in apomictic buffelgrass Kentucky bluegrass, Pennisetum squamulatum Fresen, Tripsacum L., and weeping lovegrass. Crop Sci. 37:717–723; 1997.
Rhoades, M. M. Genic control of chromosomal behavior. Maize Genet. Cooperative Newsletter 30:38–42; 1956.
Rhoades, M. M.; Dempsey, E. Induction of chromosome doubling by the elongate gene in maize. Genetics 54:505–522; 1966.
Savidan, Y. Apomixis: genetic and breeding. Plant Breed. Rev. 18:13–86; 2000.
Sheridan, W. F.; Avalkina, N. A.; Shamrov, I.-I.; Batygina T. B.; Golubovskaya, I. N. The macl gene: Controlling the commitment to the meiotic pathway in maize. Genetics 142(3):1009–1020; 1996.
Sheridan, W. F.; Golubeva, E. A.; Abrhamova, L. I.; Golubovskaya, I. The Macl mutation alters the developmental fate of the hypodermal cells and their cellular progeny in the maize anther. Genetics 153:933–941; 1999.
Siddiqui, I.; Ganesh, G.; Grossniklaus U.; Subbiah, V. The dyad gene is required for progression througth female meiosis in Arabidopsis. Development 127(1):197–207; 2000.
Skarnes, W. C. Entrapment vectors: a new tool for mammalian genetics. Bio/Technology 8:827–831; 1990.
Staiger, C. J.; Cande, W. Z. Cytoskeletal analysis of maize meiotic mutants. In: Ormrod, J. C.; Francis, D., eds., Molecular and cell biology of the plant cell cycle. Dordrecht: Kluwer Academic Publishers; 1993: 157–171.
Sumner, M. J.; van Cascele, L. Ovule development in Brassica campestris. A light microscope study. Can. J. Bot. 66:473–476; 1988.
Sundaresan, V.; Springer, P.; Volpe, T.; Haward, S.; Jones, J. D. G.; Dean, C. Ma, H.; Martienssen, R. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9:1797–1810; 1995.
Veilleux, R. Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. Plant Breed. Rev. 3:252–288; 1985.
Veronesi, F.; Mariani, A.; Bingham, E. T. Unreduced gametes in diploid Medicago and their importance in alfalfa breeding. Theor. Appl. Genet. 72:37–41; 1986.
Vielle-Calzada, J.-Ph.; Baskar, R.; Grossniklaus, U. Delayed activation of the paternal genome during seed development. Nature 404:91–94; 2000.
Vielle-Calzada, J.-Ph.; Crane, C. F.; Stelly, D. M. Apomixis: the asexual revolution. Science 274:1322–1323; 1996.
Werner, J. E.; Peloquin, S. J. Frequency and mechanisms of 2n egg formation in haploid tuberosum-wild species F1 hybrids. Am. Potato J. 64:641–654; 1987.
Willemse, M. T. M.; Van Went, J. L. The female gametophyte. In: Johri, B. M., ed. Embryology of angiosperms. New York: Springer Verlag; 1984;159–196.
Willson, C.; Pearson, R. K.; Bellen, H. J.; O'Kane, C. J.; Grossniklaus, U.; Gehring, W. J. P-element mediated enhancer detection: an efficient method for isolating and characterizing developmentally regulated genes. Genes Dev. 3:1301–1313; 1989.
Yang, W. C.; Sundaresan, V. Genetics and gametophyte biogenesis in Arabidopsis. Curr. Opin. Plant Biol. 3:53–57; 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Estrada-Luna, A.A., Huanca-Mamani, W., Acosta-García, G. et al. Beyond promiscuity: From sexuality to apomixis in flowering plants. In Vitro Cell.Dev.Biol.-Plant 38, 146–151 (2002). https://doi.org/10.1079/IVP2001278
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2001278