Abstract
Lyme disease (LD) is endemic in many regions of the Northeastern United States. Given the elusive nature of the disease, a systematic approach to identify efficient interventions would be useful for policymakers in addressing LD. We used Markov modeling to investigate the efficiency of interventions. These interventions range from awareness-based to behavioral-based strategies. Targeting animal reservoirs of LD using fungal spray or bait boxes did not prove to be an effective intervention. Results of awareness-based interventions, including distribution of signage, fliers, and presentations, implementable in different geographical scales, suggest that policymakers should focus on these interventions, as they are both cost-effective and have the highest impact on lowering LD risk. Populations may lose focus of LD warnings over time, thus quick succession of these interventions is vital. Our modeling results identify the awareness-based intervention as the most cost-effective strategy to lower the number of LD cases. These results can aid in the establishment of effective LD risk reduction policy at various scales of implementation.
Similar content being viewed by others
Introduction
Lyme disease (LD) is the most common vector-borne disease in the United States (US) currently affecting Northeastern, Mid-Atlantic, as well as Midwestern portions of the US [1, 2]. Throughout the past two decades, the number of reported LD cases in the US has risen substantially [1]. In the Northeastern US alone, the number of high-incidence counties has increased by more than 320% [3]. Various dynamic abiotic and biotic factors have allowed for the northern distribution of LD, including climate change, land use, and extent of biodiversity in an ecological landscape, coupled with resulting influences on vector and host populations [4,5,6]. Humans can acquire the infection through the bite of Ixodes scapularis or black-legged tick, that allows for transmission of Borrelia burgdorferi [7]. In the Northeastern United States, the white-footed mouse is noted as the most competent host for this bacteria [8]. Following disease transmission, early symptoms may present as prolonged fatigue, arthritis, migraines, viral-infection type symptoms, and a ‘bulls eye’ rash (erythema migrans) [9]. Although disease presentation may vary, early-stage LD detection of erythema migrans is often treated with a short course of oral antibiotics [9, 10]. While patients can experience a symptomatic improvement after initial antibiotic treatment, some may experience post-treatment symptoms of a neurologic and musculoskeletal nature, commonly referred to as post-treatment LD syndrome (PTLDS) after more than 6 months of these symptoms [10]. Patients experiencing these symptoms may receive prolonged courses of antibiotics among other potentially dangerous treatment options [10, 11].
Given the pervasive nature of the disease and its increasing geographic distribution throughout the United States [1], LD has become a major public health concern. In the US, LD imparts a total cost of up to $1.3 billion USD annually on the healthcare system, as estimated through a study investigating costs associated with LD [12]. To date, several groups have investigated the effectiveness of various LD risk reduction approaches [13, 14]. These approaches include personal protective measures (repellent use, tick check/removal, clothing modification, and tick habitat recognition), landscape modifications (leaf litter/brush reduction, mowing), vector-targeting approaches (biological and chemical acaricides, biological pesticides), mouse host targeting approaches (vaccination, antibiotic bait, tick growth regulator, bait boxes with topical acaricide) and deer host targeting approaches (fencing, reduction, vaccination, topical acaricide treatment using bait stations, and tick growth regulator) [13, 14]. Certain settings may require integrated approaches and single-intervention strategies may not address the complex dynamic between tick/host interactions and human behaviors as it relates to human LD incidence [14].
While many interventions may reduce tick abundance in a given test setting, tick abundance and tick–human interaction are not necessarily linearly correlated [15]. Thus, estimating human LD risk solely from tick abundance may not be a reliable approach, as many other human behavioral factors must be considered to better evaluate human risk [15]. In a recent study conducted by Hinckley et al. [16], tick-targeted acaricide application, known to reduce tick abundance 68–100% in controlled studies, was investigated for its potential correlation to number of reported LD cases or reported tick bites [16]. Investigators applied placebo and bifenthrin treatments in domestic landscapes, and gathered information via tick drags, surveys and medical records. While investigators recorded tick abundance to be lower on bifenthrin-treated landscapes, this occurrence was not correlated with human outcomes [16]. Bifenthrin use, as well as other chemical acaricide use may be undesirable in treating an entire domestic property due to potentially negative health and environmental consequences [13]. However, similar tick reduction effects can be achieved with biological acaricide treatment derived from fungi (Metarhizium brunneum), sold commercially as Met52 [17]. Similarly, for this approach, there was no direct connection reported between tick reduction and human LD risk [17]. A study conducted by Dolan et al. [18] investigated the impact of acaricide (fipronil) bait boxes targeting rodents. As acaricide application was limited to mouse reservoirs and other small mammals, this method may be a safer alternative to full-cover acaricide treatment while reducing tick abundance on small mammals for several weeks [18, 19].
While both fipronil bait boxes and biological acaricide treatment may not directly correlate with LD case reduction alone, there may be utility in integrating these methods to evaluate human outcomes. The Tick Project, established in 2016 by the Cary Institute of Ecosystem Studies, is currently investigating this impact [19]. In this 4-year double-blind study, the entirety of several multi-property test sites are treated with both interventions as well as different combinations of placebos [19]. Throughout this study period, the research team is actively monitoring LD incidence, tick encounters, tick and host abundance, and infection prevalence of ticks. This integrated approach may help evaluate the effectiveness of community-based tick and host-targeting interventions. Such integrated pest-management approaches, where multiple control methods are used to reduce vector populations, are a recommended initiative put forth by the United States Environmental Protection Agency (US EPA) for addressing tick-borne diseases [20]. Additionally, federal and independent researchers recommend the incorporation of public education to avoid tick habitat and the introduction of personal protective measures into an integrated pest management strategy [14, 20].
In addition to known tick-reducing agents such as tick box fipronil applications and Met52 commercial spray, integrating efforts to increase public awareness and modify risky behaviors may also prove useful. In a previously constructed model incorporating environmental and behavioral risk factors for LD, backed by ecological, demographic, and LD incidence data over a 2-year time period, model output determined that efforts to increase public awareness of risk as well as modifying risky behaviors were most effective in reducing simulated LD cases [21]. Sharareh et al. [21] simulated the effectiveness of these interventions and reported a 61% reduction in number of LD cases after applying an awareness-based intervention in 2016 and measuring the outcome in 2020. They also captured that after 2–3 years, the number of LD cases began to increase again, which may be a direct result of a population forgetting LD warnings associated with these given interventions over time.
Cost-effectiveness analysis (CEA) provides useful results in evaluating public health approaches, where CEA model parameters consider health outcomes, cost of interventions, and health-related opportunity costs [22]. Researchers have applied CEA in different settings for various public health concerns, including both clinical investigations and community-based interventions [22,23,24,25]. While ecological and behavioral knowledge gaps remain, a study of this kind may provide useful information to policymakers and researchers about the financial feasibility of integrated approaches for reducing LD risk.
To evaluate the cost-effectiveness of various LD interventions, we constructed a Markov model. A Markov model is a stochastic model that contains different states (in a healthcare scenario, health states), transition rates between these states, and the primary outputs [26, 27]. Using Markov models, we can predict the behavior of an outcome—in this investigation—the effect of a given intervention on LD cases. The core assumption of a Markov process is that the status of the states and the transition probabilities among them depend only upon the state of the system. This method is widely used in healthcare for modeling a variety of problems and formulating solution approaches [28,29,30]. Such an approach may allow for better informed healthcare policy decisions [28,29,30].
In this study, we applied CEA through the construction of a Markov model to understand the effectiveness of approaches used to reduce LD and to evaluate the cost-effectiveness of such approaches in terms of reducing the number of LD cases and other tick-borne disease (TBD) risk. We investigated the cost-effectiveness of different interventions by combining different datasets and information from ongoing and published projects.
Materials and methods
We constructed a Markov model to determine the cost-effectiveness of four different LD intervention programs, for the periods of 4 years, 10 years, and lifetime. These interventions are taken from the study by Sharareh et al. [21] and include: (1) increased population awareness over 12 months; (2) decreasing clothing risk in summer and fall; (3) mice population reduction; and (4) increasing awareness over 6 months. We compare them to the ‘Control’ or baseline program—population not affected by any treatment program. Sharareh et al. [21], adopted a simulation technique to investigate the efficacy of these interventions on reducing the LD cases. Based on their results, the awareness-based interventions were the most effective in reducing LD cases; however, behavioral-based interventions such as decreasing clothing risk could not reduce the LD cases to the awareness-based interventions’ extent. Although identifying the effective interventions is critical for the US health system, policymakers will not initiate a policy solely based on its effectiveness; efficiency must also be considered. Therefore, our main goal in this investigation is to define the cost-effective (efficient) interventions by developing a Markov model using available data sources. Our project is exempt from human subject research, thus we contacted no individuals and did not need informed consent from any.
To evaluate the cost-effectiveness of integrated host/vector targeted and individual risk reduction approaches, we scaled ecological interventions used by The Tick Project to the Binghamton University (BU), The State University of New York (SUNY) campus in Upstate New York. Here, the tick reduction approach utilized in The Tick Project is intercorrelated with the proposed mice reduction approaches used in this cost-effectiveness analysis. We adapted awareness and behavior modification interventions for the campus population from currently existing health education peer-outreach programs on the university campus, as well as from the adaptation of a current New York State Department of Health (NYS DOH) LD risk awareness initiative involving sign placement in high-risk areas. Investigators select interventions to identity the cost thresholds that would yield maximum effectiveness intensity. We selected the intervention format from a previously published article [21]. Table 1 shows the data sources along with information obtained from each. Below, we explain further our method in estimating the cost of these interventions.
We adapted the input costs for our CEA model from the existing protocol and intervention efforts, spanning both ecological and human intervention types. The ecological treatment area for this CEA includes both the main BU campus as well as trafficked areas of the adjacent nature preserve. The Tick Project protocol calls for domestic landscape feature-dependent fipronil bait box placement, however, for our analysis box placement follows manufacturer's guidelines as these parameters were better suited for the landscape features on the Binghamton University campus. According to manufacturer’s guidelines, TCS (Tick Control System)® bait boxes need to be placed every 33 feet along the length of a considered path, with an identical row placed 30–40 feet behind this row. We followed these guidelines in our cost calculations for treating nature preserve trails. Thus, we calculated treatment of 11 miles of total nature preserve trail length with 3520 boxes. For campus areas not occupied by buildings or pavement (325 acres, determined using geospatial data), manufacturer’s guidelines call for placing ten bait boxes per acre of land (3250 boxes). In total, we used 6770 bait boxes in calculation, totaling $203,100. Met52 spray application for this study follows The Tick Project protocol as these measures may be more comprehensive than manufacturer’s guidelines. We considered all organic area for treatment on the BU main campus as well as the entirety of nature preserve trails, totaling $108,519. Calculation of intervention costs are based on current research bulk pricing estimates for commercially available Met52 spray ($85/L) and TCS® bait boxes ($30/box). Reapplication protocol may be adjusted for the Tick Project study period, as protocol modifications may be ongoing throughout the study. For this present CEA study, we considered maximum intervention to identify an upper bound for intervention cost-effectiveness. Thus, reapplication of both interventions should occur at 6 months, as suggested by manufactures guidelines and The Tick Project protocol. Reapplication costs for both spray and bait box intervention total $240,534, with the spray intervention imparting the same cost as initial application and the box intervention reapplication imparting a cost only for active bait material replacement ($132,015, $19.50/box). We estimated labor costs to be $158,400, based on The Tick Project costs, that include application of both interventions and subsequent reapplication. Thus, we estimated costs calculated for ecological intervention to total $470,019, with subsequent $240,534 reapplication cost at 6 months after initial application.
The simulation study by Sharareh et al. [21], determined that the 6-month awareness intervention was more impactful in reducing LD cases as compared to the 12-month awareness intervention, with each incurring similar costs for implementation. While the 12 and 6-month awareness intervention costs are almost similar, the 6-month intervention involves a greater emphasis on peer mentors and newspaper advertisements. Here, we have calculated costs for awareness and behavior modification interventions based on a currently utilized peer-intervention program on the BU campus. This ongoing program is designed to increase health topic awareness of students as well as incite corresponding behavior modifications. This BU program, Real Education about College Health (REACH), uses peer-intervention strategies to address health topics including seasonal influenza, STD awareness, and mental health. Similar peer-intervention strategies have been cited as potentially effective tools in increasing health-related awareness and behaviors relating to mental health and diabetes [31, 32]. The REACH program on the BU Campus, incorporates different strategies for addressing a diversity of health topics. In our study, we consider the maximum intervention intensity for both awareness and behavior modification interventions. Additionally, we have considered sign posting by the NYS DOH for the awareness and behavior modification intervention calculation for the BU campus, as well as the incorporation of campus newspaper advertisements. Interventions aimed at increasing awareness in 12 months incorporated REACH program initiatives that include direct peer-intervention and literature dispersal, the production of an informational video, monthly full-page advertisements in the campus newspaper, and signposting indicating tick risk on highly trafficked BU main campus locations, totaling $41,566. We used a similar approach in assigning strategies to increase awareness in 6 months, however for this intervention we placed additional emphasis on newspaper advertisements (doubling frequency within this 6-month period), and on peer-based intervention incorporating additional costs for REACH program trained student educators.
Model structure
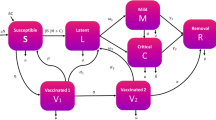
The Markov model we used in this investigation has three health states: (1) Life without LD (healthy); (2) life with LD (sick); and (3) death (Fig. 1). Patients enter the model in the healthy state. In the healthy state, each patient is given a probability of getting LD, remaining healthy or dying. In the LD state, patients either return to the health state (recover) or pass away (die). At the start of each cycle (1 year) the patient can either stay in their current state, get sick, recover, or die. Outcomes and costs are compared between treatment programs. Outcomes from the model include costs (total cost being the sum of the medical, and program costs) and quality-adjusted life years (QALYs). In this model, we assumed that any intervention has no affect on the probability of progression of LD—dying or recovering—but only in the likelihood of contracting LD.
We estimated model parameters based on the findings from a 2017 public health risk assessment study for LD [21], information listed in Table 1 and under the “Materials and methods” section, and other peer-reviewed literature as noted below. We further explain these parameters under different categories and provide them in Table 2. Using the simulation model from our reference article we calculated the transition from healthy to LD by measuring the number of individuals contracting LD per the total number of individuals susceptible to LD. The number of individuals targeted by each intervention is the population of undergraduate students at Binghamton University, as they are the only students living on the campus itself (i.e. next to the 22 walkways studied in our reference article).
Event probabilities
We estimated the probabilities of contracting LD for all four intervention programs. These estimates are based on the reference articles’ findings [21]. We simply divided the susceptible population by the number of individuals who contracted LD after applying each intervention. The probabilities of healthy recovery and LD morbidity was obtained from the existing literature [1]. These transition rates are found in Table 2.
LD costs
We obtained LD patient care costs from the published literature and have included initial hospitalization costs, pharmacy costs, and post-acute care costs [33].
Treatment costs
Treatment program costs differ greatly depending on the type of treatment and population covered by each program. We estimate the average intervention costs and treatment costs for one individual. These costs appear in Table 2 and included costs for signs, media, and extermination costs and labor, as discussed in the “Materials and methods” section. We adjusted all cost inputs to 2015 US dollars using the Consumer Price Index.
Utility inputs
We obtained utility values for healthy and LD states from the literature [33]. Here, we have assumed death to have a utility of zero.
Outcomes
Model outputs include total costs, total effectiveness, and incremental cost-effectiveness ratios (ICERs) for the first four years, ten years and lifetime. This model summed total costs across all cycles—4, 10, and lifetime. Additionally, this model measured total effectiveness as the QALY of the health state multiplied by years spent in that health state and discounted all outcomes at 3% per year. We further examined ICER sensitivity through tornado sensitivity analysis (see “Results” and “Discussion” sections). We also performed Monte Carlo risk analysis simulation to evaluate output robustness. This amounts to risk analysis by building models of possible results by substituting a range of values—a probability distribution—for any factor that has inherent uncertainty. It then calculates results over and over, each time using a different set of random values from the probability functions. Here, we have run the Monte Carlo simulation though 10,000 simulations, comparing all 4 interventions and the baseline.
Results
The results from our cost-effectiveness analysis indicate that intervention 4 is the most cost-effective intervention (see Table 3). Intervention 3 is least cost-effective and also leads to least effectiveness in reducing LD cases. In the short run (1–4 years), intervention 1 and 4 are similar. The large difference in price with only a slight change in LD prevention will cause intervention 1 to be similar to intervention 4 until year 5. After that, the difference in cost for LD cases in intervention 1 will overtake intervention 4, and intervention 4 will become significantly more cost-effective. Table 3 shows the total costs and QALYs associated with all these four interventions and the baseline (no interventions are applied).
In further analysis of CEA results, we calculate the Incremental Cost-Effectiveness Ratios (ICERs) [34,35,36] (See Table 4). Here, we define ICERs by dividing the differences in costs over the differences of QALYs, between two interventions or one intervention and the baseline (no intervention applied). Table 4 shows a unique case of ICERs where everything is either dominant (cost-saving and more effective) or dominated (cost-increasing and less effective), where we represent the reference intervention in each column, and what it is being compared against in each row.
Additionally, we have performed a tornado sensitivity analysis to determine whether the ICER results were sensitive to certain parameters in the model (Fig. 2). This approach is widely used in decision analysis to identify variables that impact model outcomes most significantly [34, 35]. In a tornado sensitivity analysis, we vary different input parameters at ± 25% of its base value, while holding other inputs constant. The horizontal bars in Fig. 2 represent the ICER range associated with the high and low values for the particular input parameter. As seen in Fig. 2, the ICER results for our CEA model are most sensitive to the Morbidity Multiplier of LD and cost of LD. This means the mortality rate and cost of treatment for LD have the largest effect on our model. We obtained both of these input parameter values from the literature. Even with large swings in these parameters, the sensitivity analysis results for Intervention 4 show that ICER values remain negative and provide evidence that the intervention is still potentially cost-effective.
Discussion
Overall, Intervention 4 is the most cost-effective intervention in every year of our analysis (Table 4). While the implementation costs of Intervention 1 are less than those for Intervention 4, Intervention 4 leads to most effective reduction in LD cases. Consequently, Intervention 4 results in the highest drop in medical cost entailed by LD cases, and thus Intervention 4 is more cost-effective then Intervention 1.
Additionally, we performed a Monte Carlo simulation analysis for robustness. We ran it through 10,000 simulations, where intervention 4 still showed as the largest potential mean cost savings per person of $6261 ($3029–$18,774). The mean QALYs per person was determined to be 3.309 (2.338–4.145).
Although this study provides a useful analysis of the efficiency of several LD interventions, there are several limitations. One limitation may be the small scale of this analysis—Binghamton University Campus—as data were only available at campus level. Another limitation may be the utilization of simulated LD cases from our reference article. While the Markov model we developed in this study is simple and does not include cost incurred from societal implications of LD, informative and complicated results are produced, capable of informing policymakers of cost-effectiveness of different healthcare strategies. Future work may consider the inclusion of societal costs in the model, such as caregiver time or productivity of patients with LD; as these factors are currently difficult to estimate and were outside the scope of this present study.
We have demonstrated a CEA model of several LD intervention strategies, using a previously established LD model along with Markov model construction. We have compiled information from established and ongoing projects to calculate estimated input costs for our model. Several CEA approaches of this type utilize approximated cost data sets for incorporation within a model, although to our knowledge no one has conducted a CEA for the LD prevention interventions investigated in this study [34,35,36,37]. Similar CEA strategies have been used by other groups to investigate treatment interventions for LD, where construction of a decision tree, obtaining estimated cost data from the literature and ongoing projects, and conducting sensitivity analysis for different model parameters are vital components [34,35,36,37]. As such, these approaches are needed to achieve valid data approximation and reliable model output for CEA efforts. The use of CEA approaches with input values obtained from the literature and ongoing projects aid in solving modern healthcare problems.
Conclusion
Information derived from these types of studies provide critical information and guidance for healthcare policy in situations where obvious approaches are not apparent. Here, our results indicate intervention 4, increasing awareness over 6 months, to be the most cost-effective approach in reducing the number of LD cases. We have conducted tornado sensitivity analysis and Monte Carlo simulation, showing support for the sensitivity of ICER values as well as the robustness of model output, respectively. This outcome suggests that state and local health officials should consider interventions to raise awareness for LD prevention in constructing an LD risk reduction approach or in corresponding resource allocation. This novel CEA model presented here can be extended to evaluate several other variations of LD interventions and also to incorporate additional healthcare and societal factors, facilitating further investigation of efficient LD intervention approaches.
References
Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for lyme disease—United States, 2008–2015. MMWR Surveill Summ. 2017;66:1.
Robinson SJ, Neitzel DF, Moen RA, et al. Disease risk in a dynamic environment: the spread of tick-borne pathogens in Minnesota, USA. EcoHealth. 2015;12:152–63.
Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 2015;21:1455–7.
Eisen RJ, Eisen L, Ogden NH, Beard CB. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J Med Entomol. 2015;53:250–61.
Wood CL, Lafferty KD. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28:239–47.
Steere A, Malawista S, Snydman D, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Reum. 1977;20:7–17.
Johnson RC, Schmid GP, Hyde FW, Steigerwalt A, Brenner DJ. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Evol Microbiol. 1984;34:496–7.
Spielman A, Levine JF, Wilson M. Vectorial capacity of North American Ixodes ticks. Yale J Biol Med. 1984;57:507.
Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134.
Feder HM Jr, Johnson BJ, O’connell S, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–30.
Marzec NS, Nelson C, Waldron PR, et al. Serious Bacterial infections acquired during treatment of patients given a diagnosis of chronic lyme disease-United States. Morb Mortal Wkly Rep. 2017;66:607–9.
Adrion ER, Aucott J, Lemke KW, Weiner JP. Health care costs, utilization and patterns of care following Lyme disease. PLoS ONE. 2015;10:e0116767.
Eisen L, Eisen RJ. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J Med Entomol. 2016;53:1050–62.
Stafford KC III, Williams SC, Molaei G. Integrated pest management in controlling ticks and tick-associated diseases. J Integr Pest Manag. 2017;8:28.
Poland GA. Prevention of Lyme disease: a review of the evidence. In: Mayo Clinic proceedings. Elsevier: Amsterdam, p. 713–24.
Hinckley AF, Meek JI, Ray JA, et al. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J Infect Dis. 2016;214:182–8.
Bharadwaj A, Stafford KC III. Evaluation of Metarhizium anisopliae strain F52 (Hypocreales: Clavicipitaceae) for control of Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2010;47:862–7.
Dolan MC, Maupin GO, Schneider BS, et al. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J Med Entomol. 2004;41:1043–54.
Keesing F, Ostfeld RS. The tick project: testing environmental methods of preventing tick-borne diseases. Trends Parasitol. 2018;34:447–50.
Beard C, Strickman D. Federal initiative: tick-borne disease integrated pest management white paper. Washington, DC: Federal Tick-Borne Disease Integrated Pest Management Workgroup; 2014.
Sharareh N, Sabounchi NS, Roome A, Spathis R, Garruto RM. Model-based risk assessment and public health analysis to prevent Lyme disease. R Soc Open Sci. 2017;4:170841.
Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 2016.
Chesson HW, Laprise J-F, Brisson M, Markowitz LE. Impact and cost-effectiveness of 3 doses of 9-valent human papillomavirus (HPV) vaccine among US females previously vaccinated with 4-valent HPV vaccine. J Infect Dis. 2016;213:1694–700.
Lo NC, Gupta R, Stanaway JD, et al. Comparison of strategies and incidence thresholds for Vi conjugate vaccines against typhoid fever: a cost-effectiveness modeling study. J Infect Dis. 2018;218:S232–42.
Gortmaker SL, Long MW, Resch SC, et al. Cost effectiveness of childhood obesity interventions: evidence and methods for CHOICES. Am J Prev Med. 2015;49:102–11.
Taylor S. Markov Models: An Introduction to Markov Models. 2017.
Noé F, Chodera J, Bowman G, Pande V, Noé F. An introduction to Markov state models and their application to long timescale molecular simulation. In: Bowman GR, et al., editors. Advances in experimental medicine and biology, vol. 797. New York: Springer; 2014.
Minion LE, Bai J, Monk BJ, et al. A Markov model to evaluate cost-effectiveness of antiangiogenesis therapy using bevacizumab in advanced cervical cancer. Gynecol Oncol. 2015;137:490–6.
Gulliford MC, Charlton J, Bhattarai N, Charlton C, Rudisill C. Impact and cost-effectiveness of a universal strategy to promote physical activity in primary care: population-based Cohort study and Markov model. Eur J Health Econ. 2014;15:341–51.
de Almeida JR, Moskowitz AJ, Miles BA, et al. Cost-effectiveness of transoral robotic surgery versus (chemo) radiotherapy for early T classification oropharyngeal carcinoma: a cost-utility analysis. Head Neck. 2016;38:589–600.
Druss BG, Zhao L, Silke A, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118:264–70.
Simmons D, Prevost AT, Bunn C, et al. Impact of community based peer support in type 2 diabetes: a cluster randomised controlled trial of individual and/or group approaches. PLoS ONE. 2015;10:e0120277.
Shadick NA, Liang MH, Phillips CB, Fossel K, Kuntz KM. The cost-effectiveness of vaccination against Lyme disease. Arch Intern Med. 2001;161:554–61.
Eschenbach TG. Spiderplots versus tornado diagrams for sensitivity analysis. Interfaces. 1992;22:40–6.
Magid D, Schwartz B, Craft J, Schwartz JS. Prevention of lyme disease after tick bites. N Engl J Med. 1992;327(8):534–41.
Lantos PM, Brinkerhoff RJ, Wormser GP, Clemen R. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector-Borne Zoonotic Dis. 2013;13(12):877–83.
Lightfoot RW, Luft BJ, Rahn DW, et al. Empirical parenteral antibiotic treatment of patients with fibromyalgia and fatigue and a positive serologic result for lyme disease: a cost-effectiveness analysis. Ann Intern Med. 1993;119:503–9.
Funding
Authors had no source of funding to conduct this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behler, R.P., Sharareh, N., Whetten, J.S. et al. Analyzing the cost-effectiveness of Lyme disease risk reduction approaches. J Public Health Pol 41, 155–169 (2020). https://doi.org/10.1057/s41271-020-00219-0
Published:
Issue Date:
DOI: https://doi.org/10.1057/s41271-020-00219-0