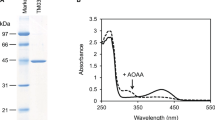
The L-lysine ɛ-aminotransferase (LAT) of Streptomyces clavuligerus was partially purified and characterized. The 51.3-kDa enzyme exhibited optimal activity at pH 7.0–7.5 and 30°C. It catalyzed transfer of the terminal amino group of L-lysine or L-ornithine to α -ketoglutarate. Oxalacetate and pyruvate were also used as acceptors of the amino group but with very low efficiency. Increasing ammonium concentrations added to chemically-defined medium MM enhanced the formation of LAT and decreased production of cephalosporins by S. clavuligerus. In cultures grown in the absence of lysine, greater enhancement of LAT formation by ammonium and less repression of cephalosporin biosynthesis were observed. In the chemically-defined GSPG medium, ammonium ions decreased cephalosporin production without showing an effect on LAT formation.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 20 August 1996/ Accepted in revised form 15 November 1996
Rights and permissions
About this article
Cite this article
Romero, J., Martín, J., Liras, P. et al. Partial purification, characterization and nitrogen regulation of the lysine ɛ-aminotransferase of Streptomyces clavuligerus . J Ind Microbiol Biotech 18, 241–246 (1997). https://doi.org/10.1038/sj.jim.2900370
Issue Date:
DOI: https://doi.org/10.1038/sj.jim.2900370