Abstract
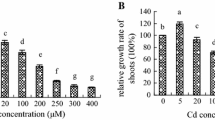
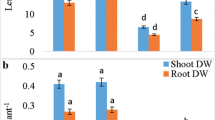
We evaluate the protective effect of nitric oxide (NO) against Cadmium (Cd) toxicity in rice leaves. Cd toxicity of rice leaves was determined by the decrease of chlorophyll and protein contents. CdCl2 treatment resulted in (1) increase in Cd content, (2) induction of Cd toxicity, (3) increase in H2O2 and malondialdehyde (MDA) contents, (4) decrease in reduced form glutathione (GSH) and ascorbic acid (ASC) contents, and (5) increase in the specific activities of antioxidant enzymes (superoxide dismutase, glutathione reductase, ascorbate peroxidase, catalase, and peroxidase). NO donors [N-tert-butyl-α-phenylnitrone, 3-morpholinosydonimine, sodium nitroprusside (SNP), and ASC + NaNO2] were effective in reducing CdCl2-induced toxicity and CdCl2-increased MDA content. SNP prevented CdCl2-induced increase in the contents of H2O2 and MDA, decrease in the contents of GSH and ASC, and increase in the specific activities of antioxidant enzymes. SNP also prevented CdCl2-induced accumulation of NH4 +, decrease in the activity of glutamine synthetase (GS), and increase in the specific activity of phenylalanine ammonia-lyase (PAL). The protective effect of SNP on CdCl2-induced toxicity, CdCl2-increased H2O2, NH4 +, and MDA contents, CdCl2-decreased GSH and ASC, CdCl2-increased specific activities of antioxidant enzymes and PAL, and CdCl2-decreased activity of GS were reversed by 2-(4-carboxy-2-phenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide, a NO scavenger, suggesting that protective effect by SNP is attributable to NO released. Reduction of CdCl2-induced toxicity by NO in rice leaves is most likely mediated through its ability to scavenge active oxygen species including H2O2.
Similar content being viewed by others
References
Anbar M. 1995. Nitric oxide: a synchronizing chemical messenger. Experientia 51: 545–550.
Beligni M.V. and Lamattina L. 1999. Nitric oxide protects against cellular damage produced by methyl viologen herbicides in potato plants. Nitric Oxide Biol. Chem. 3: 199–208.
Beligni M.V., Fath A., Bethake P.C., Lamattina L. and Jones R.L. 2002. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 129: 1642–1650.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Chaoui A., Mazhouri S., Ghorbal M.H. and Ferjani E.E. 1997. Cadmium and Zinc induction of lipid peroxidation and effects of antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 127: 139–147.
Cheng F.-Y., Hsu S.-Y. and Kao C.H. 2002.. Nitric oxide counteracts the senescence of detached rice leaves induced by dehydration and polyethylene glycol but not by sorbitol. Plant Growth Regul. 38: 265–272.
Chien H.-F. and Kao C.H. 2000. Accumulation of ammonium in rice leaves in response to excess cadmium. Plant Sci. 156: 111–115.
Chien H.-F., Lin C.C., Wang J.W., Chen C.T. and Kao C.H. 2002. Changes in ammonium ion content and glutamine synthetase activity in rice leaves caused by excess cadmium are a consequence of oxidative damage. Plant Growth Regul. 36: 41–47.
Chu C. and Lee T.M. 1989. The relationship between ethylene biosynthesis and chilling tolerance in seedlings of rice (Oryza sativa). Bot. Bull. Acad. Sin. 30: 263–273.
Clark D., Dunar J., Navarre D.A. and Klessig D.F. 2000. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol. Plant-Microbe Interact 14: 1380–1384.
d'Ischia M., Palumbo A. and Buzzo F. 2000. Interactions of nitric oxide with lipid peroxidation products under aerobic conditions: inhibitory effects on the formation of malondialdehyde and related thiobarbituric acid-reactive substances. Nitric Oxide Biol. Chem. 4: 4–14.
Foster J.G. and Hess J.L. 1980. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 66: 482–487.
Foyer C.H., Lopez-Delgado H., Dat J.F. and Scott I.M. 1997. Hydrogen peroxide and glutathione-associated mechanism of acclamatory stress tolerance and signaling. Physiol. Plant. 100: 241–254.
Gallego S.M., Benavides M.P. and Tomaro M.L. 1996. Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci. 121: 151–159.
Hahlbrock R. and Grisebach H. 1979. Enzymic controls in the biosynthesis of lignin and flavonoids. Annu. Rev. Plant Physiol. 30: 105–130.
Heath R.L. and Packer L. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125: 189–198.
Hung K.T. and Kao C.H. 2003. Nitric oxide counteracts the senescence of rice leaves induced by abscisic acid. J. Plant Physiol. 160: 871–879.
Hung K.T., Chang C.J. and Kao C.H. 2002. Paraquat toxicity is reduced by nitric oxide in rice leaves. J. Plant Physiol. 159: 159–166.
Hyodo H. and Fujinami H. 1989. The effect of 2,5-norbornadiene on the induction of the activity of 1-aminocyclopropane-1-carboxylate synthase and of phenylalanine ammonialyase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol. 30: 857–860.
Jana S. and Choudhuri M.A. 1981. Glycolate metabolism of three submerged aquatic angiosperm during aging. Aquat. Bot. 12: 345–354.
Kato M. and Shimizu S. 1987. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can. J. Bot. 65: 729–735.
Kim Y.S. and Han S. 2000. Nitric oxide protects Cu, Zn-superoxide dismutase from hydrogen peroxide-induced inactivation. FEBS Lett. 479: 25–28. ¦¦Author, please check reference not cited in text¦¦
Kumar G.N.M. and Knowles N.R. 2003. Wound-induced superoxide production and PAL activity decline with potato tuber age and wound healing ability. Physiol. Plant. 117: 108–117.
Lamattina L., Garcia-Mata C., Graziano M. and Pagnussat G. 2003. NITRIC OXIDE: the versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 54: 109–136.
Laws M.Y., Charles S.A. and Halliwell B. 1983. Glutathione and ascorbic acid in spinach chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochemical J. 210: 899–903.
Lozano-Rodriguez E., Hernandez L., Bonay P. and Charpena-Ruiz R. 1997. Distribution of cadmium in shoot and root tissue of maize and pea plants: physiological disturbances. J. Exp. Bot. 48: 123–128.
MacAdam J.W., Nelson C.J. and Sharp R.E. 1992. Peroxidase activity in the leaf elongation zone of tall fescue. Plant Physiol. 99: 872–878.
Martinez G.R., DiMascio P., Bonini M.G., Augusto O., Briviba K., Sies H., Mauer P., Röthlisberger U., Herold S. and Koppenol W.H. 2000. Peroxynitrite does not decompose to singlet oxygen ('ΔgO2) and nitroxyl (NO-). Proc. Natl Acad. Sci. USA 97: 10307–10312.
Miflin B.J. and Lea P.J. 1976. The pathway of nitrogen assimilation in plants. Phytochemistry 15: 873–885.
Nakano Y. and Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22: 867–880.
Neill S.J., Desikan R., and Hancock J.T. 2003. Nitric oxide signaling in plants. New Phytol. 159: 11–35.
Noctor G. and Foyer C.H. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 249–279.
Oaks A., Stulen J., Jones K., Winspear M.J. and Booesel I.L. 1980. Enzymes of nitrogen assimilation in maize roots. Planta 148: 477–484.
Olmos E., Martinez-Solano J.R., Piqueras A. and Hellin E. 2003. Early steps in the oxidastive burst induced by cadmium in cultured tobacco cells (BY-2 line). J. Exp. Bot. 54: 291–301.
Orozco-Cárdenas M. and Ryan C.A. 2002. Nitric oxide negatively modulate wound signaling in tomato plants. Plant Physiol. 130: 487–493.
Paoletti F., Aldinucci D., Mocali A. and Capparini A. 1986. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 154: 536–541.
Sakurai N., Katayama Y. and Yamaya T. 2001. Overlapping expression of cytosolic glutamine syntethase and phenylalanine ammonia-lyase in immature leaf blades of rice. Physiol. Plant. 113: 400–408.
Sandalio L.M., Dalurzo H.C., Gmez M., Romero-Puertas M.C. and del Río L.A. 2001. Cdamium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 52: 2115–2126.
Sanitá di Toppi L. and Gabbrielli R. 1999. Response to cadmium in higher plants. Environ. Exp. Bot. 41: 105–130.
Schützendübel A. and Polle A. 2002. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53: 1351–1365.
Schützendübel A., Schwanz P., Teichmann T., Gross K., Langenfed-Heyser R., Godbold D.L. and Polle A. 2001. Cadmium-induced changes in antioxidantive system, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 127: 887–898.
Smith I.K. 1985. Stimulation of glutathione synthesis in photo-respiring plants by catalase inhibitors. Plant Physiol. 79: 1044–1047.
Thompson J.E., Legge R.L. and Barber R.F. 1987. The role of free radical in senescence and wounding. New Phytol. 105: 317–344.
Wagner G.J. 1993. Accumulation of cadmium in crop plants and its consequence to human health. Adv. Agron. 51: 173–212.
Wink D.A., Hanbauer I., Krishna M.C., DeGraff W., Gamson J. and Mitchell J.B. 1993. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl Acad. Sci. USA 90: 9813–9817.
Wintermans J.F.G.M. and De Mots A. 1965. Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109: 448–453.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hsu, Y.T., Kao, C.H. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regulation 42, 227–238 (2004). https://doi.org/10.1023/B:GROW.0000026514.98385.5c
Issue Date:
DOI: https://doi.org/10.1023/B:GROW.0000026514.98385.5c