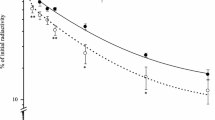
Abstract
The aim of this study was to investigate the relationship between endothelial dysfunction and low density lipoprotein (LDL) size and susceptibility to oxidation in nephrotic rats with or without deficiency of vitamin E and selenium. Four groups of male Wistar rats were studied: control (C), vitamin E and selenium deficient control (DefC), nephrotic (NS), and vitamin E and selenium deficient NS (DefNS). Nephrotic syndrome was induced by puromycin aminonucleoside. The molar ratio of vitamin E/LDL-cholesterol was significantly lower in DefNS, DefC rats, and NS vs. C rats. In comparison with control animals, vasodilation and LDL oxidability were significantly lower in nephrotic animals. LDL size was similar in all groups. Abnormal endothelial function in response to acetylcholine and carbachol was observed in NS animals compared to control rats. Relaxation response was inversely associated with an increase in LDL susceptibility to oxidation and with a lower molar ratio of vitamin E/LDL-c. LDL oxidability and LDL-c were the only variables independently associated with vasodilation. These results suggest that endothelial dysfunction of NS may be a consequence of the increased LDL susceptibility to oxidation, secondary to antioxidant deficiency.
Similar content being viewed by others
References
Harris RC, Ismail N: Extrarenal complications of the nephrotic syndrome. Am J Kidney Dis 23: 477–497, 1994
Olbricht CJ, Koch KM: Treatment of hyperlipidemia in nephrotic syndrome: Time for a change? Nephron 62: 125–129, 1992
Kaysen GA: Plasma composition in the nephrotic syndrome. Am J Nephrol 13: 347–359, 1993
Berry EM: The effects of nutrients on lipoprotein susceptibility to oxidation. Curr Opin Lipidol 3: 5–11, 1992
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320: 915–924, 1989
Witztum JL, Steinberg D: Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88: 1785–1792, 1991
Matthys KE, Van Hove CE, Kockx MM, Andries LJ, Van Osselaer N, Herman AG, Bult H: Exposure to oxidized low-density lipoprotein in vivo enhances intimal thickening and selectively impairs endothelium-dependent dilation in the rabbit. Cardiovas Res 37: 239–246, 1998
Quyyumi AA: Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 105: 32S–39S, 1998
Chait A, Brazg RL, Tribble DL, Krauss RM: Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med 94: 350–356, 1993
Liebler DC: The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol 23: 147–169, 1993
Kayden HJ, Traber MG: Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res 34: 343–358, 1993
Raij L, Nagy J, Coffee K, DeMaster EG: Hypercholesterolemia promotes endothelial dysfunction in vitamin E-and seleniumdeficient rats. Hypertension 22: 56–61, 1993
Stroes ESG, Joles JA, Chang PC, Koomans HA, Rabelink TJ: Impaired endothelial function in patients with nephrotic range proteinuria. Kidney Int 48: 544–550, 1995
Ito M, Oguri M, Naruse A, Ito H, Suzuki Y, Satake N: Impaired endothelium-dependent relaxation in isolated thoracic aorta of rats with daunomycin-induced nephrosis. J Pharmacol Exp Ther 258: 388–395, 1991
De Long DM, De Long ER, Wood PD, Lippel K, Rifkind BM: A comparison of methods for the estimation of plasma low and very low density lipoprotein cholesterol. JAMA 256: 2372–2377, 1986
Buttriss JL, Diplock AT: High performance liquid chromatography methods for vitamin E in tissues. Meth Enzymol 105: 131–138, 1984
Hatam LJ, Kayden HJ: A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res 20: 639–645, 1979
Huang CJ, Shaw HM: Tissue vitamin E status is compromised by dietary protein insufficiency in young growing rats. J Nutr 124: 571–579, 1994
Baños G, Carvajal K, Cardoso G, Zamora J, Franco M: Vascular reactivity and effect of serum in a rat model of hypertriglyceridemia and hypertension. Am J Hypertens 10: 379–388, 1997
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951
Brussaard HE, Gevers Leuven JA, Kluft C, Krans HMJ, van Duyvenvoorde W, Buytenhek R, van der Laarse A, Princen HMG: Effect of 17 β-estradiol on plasma lipids and LDL oxidation in postmenopausal women with type II diabetes mellitus. Arterioscler Thromb Vasc Biol 17: 324–330, 1997
Esterbauer H, Striegl G, Puhl H, Rotheneder M: Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Rad Res Comms 6: 67–75, 1989
Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg G: Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am J Clin Nutr 53: 314S–321S, 1991
Krauss RM, Burke DJ: Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res 23: 97–104, 1982
Ordoñez JD, Hiatt RA, Killebrew EJ, Fireman BH: The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int 44: 638–642, 1993
Joven J, Masana L, Villabona C, Vilella E, Bargalló; T, Trias M, Figueras M, Turner PR: Low density lipoprotein metabolism in rats with puromycin aminonucleoside-induced nephrotic syndrome. Metabolism 38: 491–495, 1989
Gilligan DM, Sack MN, Guetta V, Casino PR, Quyyumi AA, Rader DJ, Panza JA, Cannon RO: Effect of antioxidant vitamins on low density lipoprotein oxidation and impaired endothelium-dependent vasodilation in patients with hypercholesterolemia. J Am Coll Cardiol 24: 1611–1617, 1994
Ito M, Yamamoto I, Naruse A, Suzuki Y, Satake N, Shibata S: Impaired relaxing response to isoprenaline in isolated thoracic aorta of nephrotic rats: Decrease in release of EDRF from endothelial cells. J Cardiovasc Pharmacol 29: 232–239, 1997
Kugiyama K, Kerns SA, Morrisett JD, Roberts R, Henry PD: Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 344: 160–162, 1990
Davidge ST, Ojimba J, McLaughlin MK: Vascular function in the vitamin E-deprived rat. An interaction between nitric oxide and superoxide anions. Hypertension 31: 830–835, 1998
Wennmalm A: Endothelial nitric oxide and cardiovascular disease. J Intern Med 235: 317–327, 1994
Loscalzo J, Welch G: Nitric oxide and its role in the cardiovascular system. Progr Cardiovasc Dis 38: 87–104, 1995
Sattar N, Petrie JR, Jaap AJ: The atherogenic lipoprotein phenotype and vascular endothelial dysfunction. Atherosclerosis 138: 229–235, 1998
Chin JH, Azhar S, Hoffman BB: Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J Clin Invest 89: 10–18, 1992
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Posadas-Romero, C., Posadas-Sánchez, R., Zamora-González, J. et al. LDL size and susceptibility to oxidation in experimental nephrosis. Mol Cell Biochem 220, 61–68 (2001). https://doi.org/10.1023/A:1010874306937
Issue Date:
DOI: https://doi.org/10.1023/A:1010874306937