Abstract
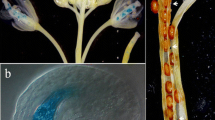
The oilseed rape (Brassica napus) endo-polygalacturonase (endo-PG) RDPG1 is involved in middle lamella breakdown during silique opening. We investigated tissue-specific expression of RDPG1 in transgenic Arabidopsis thaliana. Cellular localization of endo-PG protein in Arabidopsis siliques was determined by immuno-electron microscopy. An Arabidopsis orthologue, ADPG1, was isolated and aligned with the sequence of RDPG1. The proximal 5′ sequences as well as introns are largely conserved. Analysis of the histological GUS-staining pattern of two RDPG1 promoter-GUS (β-glucuronidase) constructs in transgenic Arabidopsis revealed that the conserved proximal part of the 5′-flanking region directs expression in dehiscence zones of siliques and anthers, floral abscission zones and stylar tissues during pollen tube growth, branch points between stems and pedicel and expression associated with the apical meristem of seedlings, while the distal part of theRDPG1 5′-flanking region contains elements involved in vascular-associated expression in petals, cotyledons and roots. Subsequent RT-PCR analysis, on RNA from the corresponding rape tissues, confirms the staining pattern revealed in transgenic Arabidopsis, thereby justifying the use of Arabidopsis as a reliable model system for analysis of oilseed rape regulatory sequences.
Similar content being viewed by others
References
Allen, R.L. and Lonsdale, D.M. 1993. Molecular characterisation of one of the maize polygalacturonase gene family members which are expressed during late pollen development. Plant J. 3: 261–271.
Bechtold, N., Ellis, J. and Pelletier, G. 1993. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. Life Sci. 316: 1194–1199.
Bent, A.F., Kunkel, B.N., Dahlbeck, D., Schmidt, R., Giraudat, J., Leung, J. and Staskawicz, B.J. 1994. RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265: 1856–1860.
Deikman, J., Kline, R. and Fischer, R.L. Organization of ripening and ethylene regulatory regions in a fruit-specific promoter from tomato (Lycopersicum esculentum). Plant Physiol. 100: 2013–2017.
Del Campillo, E., Reid, P.D., Sexton, R. and Lewis, L.N. 1990. Occurrence and localization of 9.5 cellulase in abscising and non-abscising tissues. Plant Cell 2: 245–254.
De Silva, J., Jarman, C.D., Arrowsmith, D.A., Stronach, M.S., Chengappa, S., Sidebottom, C. and Reid, J.S.G. 1993. Molecular characterisation of a xyloglucan-specific endo-(1→4)-β-D-glucanase (xyloglucan endo-transglycosylase) from nasturtium seeds. Plant J. 3: 701–711.
Fischer, R.L. and Bennett, A.B. 1991. Role of cell wall hydrolases in fruit ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42: 675–703.
Hajdukiewicz, P., Svab, Z. and Maliga, P. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25: 989–994.
Hong, H.P., Gerster, J.L., Datla, R.S.S., Albani, D., Scoles, G., Keller, W. and Robert, L.S. 1997. The promoter of a Brassica napus polygalacturonase gene directs pollen expression of β-glucuronidase in transgenic Brassica plants.Plant Cell Rep. 16: 373–378.
Jefferson, R.A., Kavanagh, T.A. and Bevan, M.W. 1987. β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907.
Jenkins, E.S., Paul, W., Coupe, S.A., Bell, S.J., Davies, E.C. and Roberts, J.A. 1996. Characterization of an mRNA encoding a polygalacturonase expressed during pod development in oilseed rape (Brassica napus L.) J. Exp. Bot. 47: 111–115.
Jenkins, E.S., Paul, W., Craze, M., Whitelaw, C.A., Weigand, A. and Roberts, J.A. 1999. Dehiscence-related expression of an Arabidopsis thaliana gene encoding a polygalacturonase in transgenic plants of Brassica napus. Plant Cell Envir. 22: 159–167.
Kalaitzis, P., Koehler, S.M. and Tucker, M.L. 1995. Cloning of a tomato polygalacturonase expressed in abscission. Plant Mol. Biol. 28: 647–656.
Kalaitzis, P., Solomos, T. and Tucker, M.L. 1997. Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol. 113: 1303–1308.
Koehler, S.M., Matters, G.L., Nath, P., Kemmerer, E.C. and Tucker, M.L. 1996. The gene promoter for a bean abscission cellulase is ethylene-induced in transgenic tomato and shows high sequence conservation with a soybean abscission cellulase. Plant Mol. Biol. 31: 595–606.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Lashbrook, C.C., Gonzales-Bosch, C. and Bennett, A.B. 1994. Two divergent endo-∃-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 6: 1485–1493.
Meakin, P.J. and Roberts, J.A. 1990. Dehiscence of fruit in oilseed rape (Brassica napus L.). I. Anatomy of pod dehiscence. J. Exp. Bot. 41: 995–1002.
Medford, J.I., Elmer, J.S. and Klee, H.J. 1991. Molecular cloning and characterization of genes expressed in shoot apical meristems. Plant Cell 3: 359–370
Montgomery, J., Pollard, V., Deikman, J. and Fischer, R.L. 1993. Positive and negative regulatory regions control the spatial distribution of polygalacturonase transcription in tomato fruit pericarp. Plant Cell 5: 1049–1062.
Moran, R. 1982. Formulae for determination of chlorophyllous pigments extracted with N,N -dimethylformamide. Plant Physiol. 69: 1376–1381.
Nicholas, F.J., Smith, C.J.S., Schuch, W., Bird, C.R. and Grierson, D. 1995. High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol. Biol. 28: 423–435.
Petersen, M., Sander, L., Child, R., van Onckelen, H., Ulskov, P. and Borkhardt, B. 1996. Isolation and characterization of a pod dehiscence zone specific polygalacturonase from Brassica napus. Plant Mol. Biol. 31: 517–527.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
Sander, L., Botterman, J., Ulvskov, P. and Borkhardt, B. 1996. Nucleotide sequence of a gene encoding a pod dehiscence zone specific endo-polygalacturonase (Acc. no. X98373) from Brassica napus. Plant Gene Register 96-056. Plant Physiol. 111: 1354.
Sanger, F., Nicklen, S. and Coulsen, A.R. 1977. DNA sequencing with chain termination inhibitors. Proc. Natl. Acad. Sci. USA 74: 5463–5467.
Schröder, M., Dixelius, C., Rahlen, L. and Glimelius, K. 1994. Transformation of Brassica napus by using the aadA gene as selectable marker and inheritance studies of the marker genes. Physiol. Plant. 92: 37–46.
Sexton, R., Durbin, M.L., Lewis, L.N. and Thomson, W.W. 1980. Use of cellulase antibodies to study leaf abscission. Nature 283: 873–874.
Spence, J., Vercher, Y., Gates, P. and Harris, N. 1996. Pod shatter in Arabidopsis thaliana, Brassica napus and B. juncea.J.Microsc. 181: 195–203.
Svab, Z., Harper, E., Jones, J. and Maliga, P. 1990. Aminoglycoside-3′-adenyltransferase confers resistance to spectinomycin and streptomycin in Nicotiana tabacum. Plant Mol. Biol. 14: 197–205.
Tebbutt, S.J., Rogers, J.H. and Lonsdale, D.M. 1994. Characterization of a tobacco gene encoding a pollen-specific polygalacturonase. Plant Mol. Biol. 25: 283–297.
Tonutti, P., Cass, L.G. and Christoffersen, R.E. 1995. The expression of cellulase gene family members during induced avocado fruit abscission and ripening. Plant Cell Envir. 18: 709–713.
Tucker, M.L., Baird, S.L. and Sexton, R. 1991. Bean leaf abscission: tissue specific accumulation of a cellulase mRNA. Planta 186: 52–57.
Xu, W., Purugganan, M.M., Polisensky, D.H., Antosiewich, D.M., Fry, S.C. and Braam, J. 1995. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotrans-glycosylase. Plant Cell 7: 1555–1567.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sander, L., Child, R., Ulvskov, P. et al. Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol Biol 46, 469–479 (2001). https://doi.org/10.1023/A:1010619002833
Issue Date:
DOI: https://doi.org/10.1023/A:1010619002833