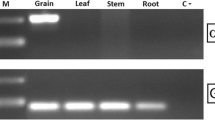
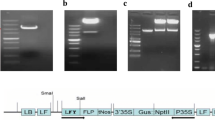
Abstract
Seedlessness is a highly desirable characteristic in fresh fruit. Marketability of a fruit as seedless does not require complete absence of seeds as long as the seed structures are imperceptible during consumption. Chimaeric genes comprised of soybean β-conglycinin seed storage protein gene promoters linked to the bacterial RNase gene, Barnase, were tested for their efficacy to cause seed death and decrease seed size in tobacco and Arabidopsis. These species were used because they undergo two distinct seed developmental pathways and produce albuminous and exalbuminous seeds, respectively. In both species, the death of embryo and endosperm tissues occurred, resulting in a dominant seed lethal phenotype with segregation distortion. Reduction in seed size was only observed in Arabidopsis seeds and the phenotype resembled that of stenospermocarpic seeds in grape. Some transformants of both species were male-sterile and this correlated with the expression of the gene in anthers indicating that expression of the gene is not strictly seed-specific. The promoters also direct expression of a linked GUS gene to Citrus embryos of various developmental stages, and Citrus forms exalbuminous seeds, therefore, the Barnase constructions may be useful in eliciting a reduction in seed size of around 75% of the seeds found in the fruit. This may be sufficient to warrant marketing as ‘less seedy’ if trials in the cultivar of interest indicate that the smaller seeds are less detectable to the consumer. Abbreviations: GUS, β-glucuronidase; PCR, polymerase chain reaction; DMSO, dimethyl sulfoxide; DTA, diphtheria toxin-A chain; CFDA, 5(6)-carboxy-fluorescein di-acetate; CG, β-conglycinin; DAP, days after pollination; FAA, formaldehyde-acetic acid alcohol fixative.
Similar content being viewed by others
References
An G: Binary Ti vectors for plant transformation and promoter analysis. Meth Enzymol 153: 292–305 (1987).
Avery GS Jr: Structure and germination of tobacco seed and the developmental anatomy of the seedling plant. Am J Bot 20: 309–327 (1933).
Barker SJ: Developmental regulation of soybean (-conglycinin gene expression. Ph D thesis, University of California, Los Angeles, CA (1990).
Barker SJ, Harada JJ, Goldberg RB: Cellular localization of soybean storage protein mRNA in transformed tobacco seeds. Proc Natl Acad Sci USA 85: 458–462 (1988).
Catsimpoolas N, Campbell TG, Meyer EW: Immunochemical study of changes in reserve proteins of germinating soybean seeds. Plant Physiol 43: 799–805 (1968).
Czako M, Jang J-C, Herr JM Jr, Marton L: Differential manifestation of seed mortality induced by seed-specific expression of the gene for diphtheria toxin A chain in Arabidopsis and tobacco. Mol Gen Genet 235: 33–40 (1992).
Denis MR, Delourme R, Gourret J-P, Mariani C, Renard M: Expression of engineered male sterility in Brassica napus. Plant Physiol 101: 1295–1304 (1993).
Doyle JJ, Schuler MA, Godette WD, Zenger V, Beachy RN, Slightom JL: The glycosylated seed storage proteins of Glycine max and Phaseolus vulgaris. J Biol Chem 261: 9228–9238 (1986).
Erdelska O: Dynamics of the development of embryo and endosperm I [Papaver somniferum, Nicotiana tabacum, Jasione montana]. Biologia (Bratislava) 40: 17–30 (1985).
Erdelska O: Cytolysis of the endosperm in different types of correlation in endosperm and embryo development. Acta Bot Neerl 35: 437–441 (1986).
Finer JJ, Vain P, Jones MW, McMullen MD: Development of the particle inflow gun for DNA delivery into plant cells. Plant Cell Rep 11: 323–328 (1992).
Gillaspy G, Ben-David H, Gruissem W: Fruits: a developmental perspective. Plant Cell 5: 1439–1451 (1993).
Goldberg RB, Hoschek G, Tam SH, Ditta GS, Breidenbach RW: Abundance, diversity and regulation of mRNA sequence sets in soybean embryogenesis. Devel Biol 83: 201–217 (1981).
Goldman MHS, Goldberg RB, Mariani C: Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO J 13: 2976–2984 (1994).
Harada JJ, Barker SJ, Goldberg RB: Soybean β-conglycinin genes are clustered in several DNA regions and are regulated by transcriptional and post-transcriptional processes. Plant Cell 1: 415–425 (1989).
Hartley RW: Barnase and barstar. Expression of its cloned inhibitor permits expression of a cloned ribonuclease. J Mol Biol 202: 913–915 (1988).
Hidaka T, Omura M: Control of embryogenesis in Citrus cell culture: regeneration from protoplasts and attempts to callus bank. Bull Fruit Tree Res Stn B16: 1–17 (1989).
Hidaka T, Omura M, Ugaki M, Tomiyama M, Kato A, Ohshima M, Motoyoshi F: Agrobacterium-mediated transformation and regeneration of Citrus spp. from suspension cells. Jpn J Breed 40: 199–207 (1990).
Higgins TJV, Newbigin EJ, Spencer D, Llewellyn DJ, Craig S: The sequence of a pea vicilin gene and its expression in transgenic tobacco plants. Plant Mol Biol 11: 683–695 (1988).
Hirai MY, Fujiwara T, Goto K, Komeda Y, Chino M, Naito S: Differential regulation of soybean seed storage protein gene promoter-GUS fusions by exogenously applied methionine in transgenic Arabidopsis thaliana. Plant Cell Physiol 35: 927–934 (1994).
Horsch RB, Fry JE, Hoffman NL, Eicholtz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase is a sensitive and versatile fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK: Control of Arabidopsis flower and seed development by the homeotic gene apetela-2. Plant Cell 6: 1211–1225 (1994).
Kandasamy MK, Thorsness MK, Rundle SJ, Goldberg ML, Nasrallah JB, Nasrallah ME: Ablation of papillar cell function in Brassica flowers results in the loss of stigma receptivity to pollination. Plant Cell 5: 263–275 (1993).
Kim Y, An G: Pollen-specific expression of the Arabidopsis thaliana α 1-tubulin promoter assayed by β-glucuronidase, chloramphenicol acetyltransferase and diphtheria toxin reporter genes. Transgen Res 1: 188–194 (1992).
Klein TM, Gradziel T, Fromm ME, Sanford JC: Factors influencing gene delivery into Zea mays cells by high velocity microprojectiles. Bio/technology 6: 559–563 (1988).
Koltunow AM: Isolation and construction of genes to control seed production in Citrus. In: Hayashi T, Omura M, Scott NS (eds) Techniques on Gene Diagnosis and Breeding in Fruit Trees, pp. 101–108. Fruit Tree Research Station, Tsukuba, Japan (1993).
Koltunow AM, Brennan P: Paternal transmission of a seed size reduction gene varies with age of a primary transformant and seed set is also influenced by gene expression in maternal tissues. Mol Breed 4: 253–261 (1998).
Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB: Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2: 1201–1224 (1990).
Koltunow AM, Soltys K, Nito N, Mc Clure S: Anther, ovule, seed and nucellar embryo development in Citrus sinensis cv. Valencia. Can J Bot 73: 1567–1582 (1995).
Koltunow AM, Hidaka T, Robinson SP: Polyembryony in Citrus: accumulation of seed storage proteins in seeds and embryos cultured in vitro. Plant Physiol 110: 599–609 (1996).
Konieczny A, Ausubel FM: A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403–410 (1993).
Koning, A, Jones A, Fillatti J, Comai L, Lassner MW: Arrest of embryo development in Brassica napus mediated by modified Pseudomonas aeruginosa exotoxin A. Plant Mol Biol 18: 247–258 (1992).
Ledbetter CA, Ramming DW: Seedlessness in grapes. In: Janick J (ed) Horticultural Reviews, pp. 159–184. Timber Press, Portland Oregon (1989).
Lee TD: Patterns of fruit and seed production. In: Doust JL, Doust LL (eds) Plant Reproductive Ecology: Patterns and Strategies, pp. 179–202. Oxford University Press, Oxford (1987).
Leon-Kloosterziel KM, Keijzer CJ, Koornneef MA: A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell 6: 385–392 (1994).
Luckwill LC: Fruit growth in relation to external and internal chemical stimuli. In: Rudnick D (ed) Cell, Organism and Milieu, pp. 223–251. Ronald Press, New York (1959).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1982).
Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg R.B: Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature 347: 737–741 (1990).
Mariani C, Gossele V, De Beuckeleer M, De Block M, Goldberg RB, De Greef W, Leemans J: A chimaeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature 357: 384–387 (1992).
Meinke DW, Sussex IM: Embryo-lethal mutants of Arabidopsis thaliana. A model system for genetic analysis of plant embryo development. Devel Biol 72: 50–61 (1979).
Moore GA, Jacono CC, Neidigh JL, Lawrence SD, Cline K: Agrobacterium-mediated transformation of stem segments and regeneration of transgenic plants. Plant Cell Rep 11: 238–242 (1992).
O'Brien TP, McCully ME: The study of plant structure. Principles and selected methods. Termacarphi Press, Australia (1981).
Pearson HM: Parthenocarpy and seed abortion in Vitis vinifera. Proc Am Soc Hort Sci 29: 169–175 (1932).
Pena L, Cervera M, Juarez J, Navarro A, Pina JA, Duran-Vila N, Navarro L: Agrobacterium-mediated transformation of sweet orange and regeneration of transgenic plants. Plant Cell Rep 14: 616–619 (1995).
Quiggin D: Transcriptional regulation of a pea vicilin gene. Ph D thesis, Australian National University, Canberra, ACT, Australia. (1993)
Reynaerts A, Van de Wiele H, De Sutter G, Janssens J: Engineered genes for fertility control and their application in hybrid seed production. Sci Hort 55: 125–139 (1993).
Roth I: Fruits of angiosperms. Borntraeger, Berlin (1977).
Sano M, Kawashima N: Isolation and partial characterization of the major seed protein from Nicotiana tabacum and accumulation during development. Agric Biol Chem 47: 1305–1310 (1983).
Schuler MA, Schmitt ES, Beachy RN: Closely related families of genes code for the α and α′ subunits of the soybean 7S storage protein complex. Nucl Acids Res 10: 8225–8244 (1982).
Spurr AP: A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43 (1969).
Stout AB: Seedlessness in grapes. New York State Agric Exp Stn (Geneva) Tech Bul 238 (1936).
Sykes SR, Possingham JV: The effect of excluding insect pollinators on seediness of Imperial mandarin fruits. Aust J Exp Agric 32: 409–411 (1992).
Sykes SR, Koltunow AM, Lee LS: The genetic improvement of mandarins in Australia. Aust Citrus News 70: 8–12 (1994).
Thanh VH, Shibasaki K: Heterogeneity of β-conglycinin. Biochim Biophys Acta 439: 326–338 (1976).
Thorsness MK, Kandasamy MK, Nasrallah ME, Nasrallah JB: Genetic ablation of floral cells in Arabidopsis. Plant Cell 5: 253–261 (1993).
Valvekens D, Van Montagu M, Lijsebettens M: Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 85: 5536–5540 (1988).
van der Geest AHM, Frisch DA, Kemp JD, Hall TC: Cell ablation reveals that expression from the phaseolin promoter is confined to embryogenesis and microsporogenesis. Plant Physiol 109: 1151–1158 (1995).
Weber H, Borisjuk L, Wobus U: Sugar import and metabolism during seed development. Trends Plant Sci 2: 169–174 (1997).
Weinberger JH, Harmon FN: Seedlessness in vinifera grapes. Proc Am Soc Hort Sci 85: 270–274 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koltunow, A.M., Brennan, P., Bond, J.E. et al. Evaluation of genes to reduce seed size in shape Arabidopsis and tobacco and their application to shape Citrus. Molecular Breeding 4, 235–251 (1998). https://doi.org/10.1023/A:1009610819338
Issue Date:
DOI: https://doi.org/10.1023/A:1009610819338