Abstract
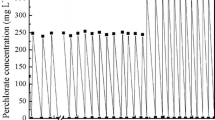
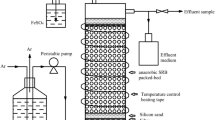
The removal of carbon tetrachloride under sulfate reducing conditions was studied in an an aerobic packed-bed reactor. Carbon tetrachloride, up to a concentration of 30 μM, was completely converted. Chloroform and dichloromethane were the main transformation products, but part of the carbon tetrachloride was also completely dechlorinated to unknown products. Gram-positive sulfate-reducing bacteria were involved in the reductive dechlorination of carbon tetrachloride to chloroform and dichloromethane since both molybdate, an inhibitor of sulfate reduction, and vancomycin, an inhibitor of gram-positive bacteria completely inhibited carbon tetrachloride transformation. Carbon tetrachloride transformation by these bacteria was a cometabolic process and depended on the input of an electron donor and electron acceptor (sulfate). The rate of carbon tetrachloride transformation by sulfate reducing bacteria depended on the type of electron donor present. A transformation rate of 5.1 nmol·ml-1·h-1 was found with ethanol as electron donor. At carbon tetrachloride concentrations higher than18 μM, sulfate reduction and reductive dechlorination of carbon tetrachloride decreased and complete inhibition was observed at a carbon tetrachloride concentration of 56.6 μM. It is not clear what type of microorganisms were involved in the observed partial complete dechlorination of carbon tetrachloride. Sulfate reducing bacteria probably did not play a role since inhibition of these bacteria with molybdate had no effect on the complete dechlorination of carbon tetrachloride.
Similar content being viewed by others
References
Bouwer EJ & McCarty PL (1983) Transformations of 1-and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl. Environ. Microbiol. 45: 1286–1294
Bouwer EJ & McCarty PL (1983a) Transformations of halogenated organic compounds under denitrification conditions. Appl. Environ. Microbiol. 45: 1295–1299
Bouwer EJ & Wright JP (1988) Transformations of trace halogenated aliphatics in anoxic biofilm column. J. Cont. Hydrol. 2: 155–169
Chiu P-C & Reinhard M (1996) Transformation of carbon tetrachloride by reduced vitamin B12 in aqueous cysteine solution. Environ. Sci. Technol. 30: 1882–1889
Cobb GD & Bouwer EJ (1991) Effects of electron acceptors on halogenated organic compound biotransformations in a biofilm column. Environ. Sci. Technol. 25: 1068–1074
Criddle CS, DeWitt JT, Grbic-Galic D & McCarty PL (1990) Transformation of carbon tetrachloride by Pseudomonassp. strain KC under denitrification conditions. Appl. Environ. Microbiol. 56: 3240–3246
Criddle CS & McCarty PL (1991) Electrolytic model system for reductive dehalogenation in aqueous environments. Environ Sci Technol 25: 973–978
Curtis GP & Reinhard M (1994) Reductive dehalogenation of hexachloroethane, carbon tetrachloride, and bromoform by anthrahydroquinone disulfonate and humic acid. Environ. Sci. Technol. 28: 2393–2401
de Best JH, Jongema H, Weijling A, Doddema HJ Janssen DB & Harder W (1997) Transformation of 1,1,1-trichloroethane in an anaerobic packed-bed reactor at various concentrations of 1,1,1-trichloroethane, acetate and sulfate. Appl. Microbiol. Biotechnol. 48: 417–423
Distefano TD, Gossett JM & Zinder SH (1992) Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl. Environ. Microbiol. 58: 3622–3629
Egli C, Scholtz R, Cook AM & Leisinger T (1987) Anaerobic dechlorination of tetrachloromethane and 1,2-dichloroethane to degradable products by pure cultures of Desulfobacteriumsp. and Methanobacteriumsp. FEMS Microbiol. Lett. 43: 257–261
Egli C, Tschan T, Scholtz R, Cook AM & Leisinger T (1988) Transformation of tetrachloromethane to dichloromethane and carbon-dioxide by Acetobacterium woodii. Appl. Environ. Microbiol. 54: 2819–2824
Egli C, Stromeyer SA, Cook AM & Leisinger T (1990) Transformation of tetrachloromethane and chloroform to CO2 by anaerobic bacteria is a non-enzymic process. FEMS Microbiol. Lett. 68: 207–212
Gälli R & McCarty PL (1989) Biotransformation of 1,1,1-trichloroethane, trichloromethane, and tetrachloromethane by a Clostridiumsp.. Appl. Environ. Microbiol. 55: 837–844
Hashham S, Scholze R & Freedman DL (1995) Cobalamin-enhanced anaerobic transformation of carbon tetrachloride. Environ. Sci. Technol. 29: 2856–2863 Kriegman-King MR & Reinhard M (1992) Transformation of carbon tetrachloride in the presence of sulfide, biotite and vermiculite. Environ. Sci. Technol. 26: 2198-2206
Lewis TA & Crawford RL (1993) Physiological factors affecting carbon tetrachloride dehalogenation by the denitrifying bacterium Pseudomonassp. strain KC. Appl. Environ. Microbiol. 59: 1635–1641
Mägli A, Wendt M & Leisinger T (1996) Isolation and characterization of Dehalobacterium formicoaceticumgen. nov. sp. nov., a strictly anaerobic bacterium utilizing dichloromethane as source of carbon and energy. Arch. Microbiol. 166: 101–108
Mikesell MD & Boyd SA (1990) Dechlorination of chloroform by Methanosarcinastrains. Appl. Environ. Microbiol. 56: 1198–1201
Picardal FW, Arnold RG, Couch H, Little AM & Smith ME (1993) Involvement of cytochromes in the anaerobic biotransformation of tetrachloromethane by Shewanella putrefaciens200. Appl. Environ. Microbiol. 59: 3763–3770
Picardal FW, Arnold RG & Huey BB (1995) Effects of electron donor and acceptor conditions on reductive dehalogenation of tetrachloromethane by Shewanella putrefaciens200. Appl. Environ. Microbiol. 61: 8–12
Smith RL & Klug MJ (1981) Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl. Environ. Microbiol. 42: 116–121
Stromeyer SA, Stumpf K, Cook AM & Leisinger T (1992) Anaerobic degradation of tetrachloromethane by Acetobacterium woodii: separation of dechlorinative activities in cell extracts and roles for vitamin B12 and other factors. Biodegradation 3: 113–123
Thauer RK, Jungermann K & Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41: 100–180
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Best, J.H., Salminen, E., Doddema, H.J. et al. Transformation of carbon tetrachloride under sulfate reducing conditions. Biodegradation 8, 429–436 (1997). https://doi.org/10.1023/A:1008262225760
Issue Date:
DOI: https://doi.org/10.1023/A:1008262225760