Abstract
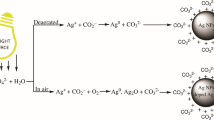
Ultraviolet (UV) photoirradiation of Ag(I) compounds in the presence of an aqueous Triton X-100 solution has been exploited for the first time to prepare reproducible yellow silver hydrosol. The evolution of nanosized silver particles has been examined critically under the influence of different anions/ligands. Hence, time dependent evolution of silver hydrosol from different silver compounds in micelle via photochemical reduction is observed. Anions/ligands of precursor salts have been found to show profound influence (due to electron scavenging property, solubility, stability etc.) on the evolution route and efficiency of photochemical reduction of Ag(I) to Ag(O) in micelle and thereby classification of silver compounds becomes possible. Kinetic results reveal that the formation of silver particles proceeds via autocatalytic growth mechanism. The observed variation in rate constant values for the evolution of nanoparticles from different silver compounds have been explained in terms of available thermodynamic and kinetic parameters. Nucleophile induced dissolution and reversible photogeneration of zerovalent silver particles have been investigated under ambient condition.
Similar content being viewed by others
References
Abid J.P., A.W. Wark, P.F. Brevet & H.H. Girault, 2002. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. J. Chem. Soc. Chem. Commun. 792–793.
Alivisatos A.P., 1996. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. B 100, 13226–13239.
Belloni J., M. Mostafavi, H. Remita, J.L. Marignier & M.O. Delcourt, 1998. Radiation-induced synthesis of monoand multi-metallic clusters and nanocolloids. NewJ. Chem. 22, 1239–1255.
Brust M., M. Walker, D. Bethel, D.J. Schiffrin & R. Whyman, 1994. Synthesis of thiol-derivatized gold nanoparticles in a twophase liquid–liquid system. J. Chem. Soc. Chem. Commun. 801–802.
Cheng H. & U. Landman, 1994. Controlled deposition and glassification of copper nanoclusters. J. Phys. Chem. 98, 3527–3537.
Esumi K., T. Hosoya, A. Suzuki & K. Torigoe, 2000. Spontaneous formation of gold nanoparticles in aqueous solution of sugarpersubstituted poly(amidoamine) dendrimers. Langmuir 16, 2978–2980.
Eychmüller A., 2000. Structure and photophysics of semiconductor nanocrystals. J. Phys. Chem. B 104, 6514–6528.
Goia D.V. & E. Matijević, 1998. Preparation of monodispersed metal particles. New J. Chem. 22, 1203–1215.
Gutiérrez M. & A. Henglein, 1993. Formation of colloidal silver by ‘push–pull’ reduction of silver(1+). J. Phys. Chem. 97, 11368–11370.
Heath J.R. & J.J. Shiang, 1998. Covalency in semiconductor quantum dots. Chem. Soc. Rev. 65–71.
Henglein A., 1993. Physicochemical properties of small metal particles in solution: ‘Microelectrode’ reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 97, 5457–5471.
Henglein A., 1998. Radiolytic control of the size of colloidal gold nanoparticles. Langmuir 14, 7392–7396.
Huang Z.Y., G. Mills & B. Hajek, 1993. Spontaneous formation of silver particles in basic 2-propanol. J. Phys. Chem. 97, 11542–11550.
Jin R., Y.W. Cao, C.A. Mirkin, K.L. Kelly, G.C. Schatz & J.G. Zheng, 2001. Photoinduced conversion of silver nanospheres to nanoprisms. Science 294, 1901–1903.
Kamat P.V., 1993. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem. Rev. 93, 267–300.
Kamat P.V., 1997. In: Kamat P.V. and Miesel D. eds. Semiconductor Nanoclusters – Physical, Chemical and Catalytic Aspects. Elsevier Science, Amsterdam, pp. 237–259.
Kamat P.V., M. Flumiani & G.V. Hartland, 1998. Picosecond dynamics of silver nanoclusters photoejection of electrons and fragmentation. J. Phys. Chem. B 102, 3123–3128.
Kreibig U. & M. Vollmer, 1995. Optical Properties of Metal Clusters. Springer, Berlin.
Kreibig U., M. Gartz, A. Hilger & H. Hovel, 1996. In: Pelizzatti E. ed. Fine Particles Science and Technology. Kluwer Academic Publishers, Boston, p. 499.
Lawless D., S. Kapoor, P. Kennephol, D. Miesel & N. Serpone, 1994. Reduction and aggregation of silver ions at the surface of colloidal silica. J. Phys. Chem. 98, 9619–9625.
Link S. & M.A. El-Sayed, 1999a. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 103, 4212–4217.
Link S. & M. A. El-Sayed, 1999b. Spectral properties and relaxation dynamics of surface plaE. Pelizzattismon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 103, 8410–8426.
Manna L., E.C. Scher & A.P. Alivisatos, 2000. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 122, 12700–12706.
Mie G., 1908. Contributions to the optics of turbid media, especially colloidal metal solutions. Ann. Phys. 25, 377–445.
Mizukoshi Y., K. Okitsu, Y. Maeda, T.A. Yamamoto, R. Oshima & Y. Nagata, 1997. Sonochemical preparation of bimetallic nanoparticles of gold/palladium in aqueous solution. J. Phys. Chem. B 101, 7033–7037.
Mostafavi M. & J. Belloni, 1997. Ligand-dependent properties of transient and long-lived metal clusters in solution. Recent Res. Develop. Phys. Chem. 1, 459–474.
Mulvaney P., M. Giersig & A. Henglein, 1993. Electrochemistry of multilayer colloids: Preparation and absorption spectrum of gold-coated silver particles. J. Phys. Chem. 97, 7061–7064.
Mulvaney P., 1996. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12, 788–800.
Mulvaney P., 1997. In: Kamat P.V. and Miesel D. eds. Semiconductor Nanoclusters – Physical, Chemical and Catalytic Aspects. Elsevier Science, Amsterdam, p. 99.
Mulvaney P., L.M. Liz-Marzan, M. Giersig & T. Ung, 2000. Silica encapsulation of quantum dots and metal clusters. J. Mater. Chem. 10, 1259–1270.
Nirmal M., B.O. Dabbousi, M.G. Bawendi, J.J. Macklin, J.K. Trautman, T.D. Harris & L.E. Brus, 1996. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature 383, 802–804.
Ostwald W., 1901. The history of colloidal gold. Z. Chem. Ind. Kolloide 4(1909), 5–14.
Pal A., 1998. Photoinduced gold sol generation in aqueous Triton X-100 and its analytical application for spectrophotometric determination of gold. Talanta 46, 583–587.
Pal T., A. Ganguly & D.S. Maity, 1986. Determination of cyanide based upon its reaction with colloidal silver in the presence of oxygen. Anal. Chem. 58, 1564–1566.
Pileni M.P., 1998. Optical properties of nanosized particles dispersed in colloidal solutions or arranged in 2D or 3D superlattices. New J. Chem. 22, 693–702.
Pileni M.P., 2001. Nanocrystal self-assemblies: Fabrication and collective properties. J. Phys. Chem. B 105, 3358–3371.
Privman V., D.V. Gioa, P. Jongsoon & E. Matijevié 1999. Mechanism of formation of monodispersed colloids by nanosize precursors. J. Colloid Interface Sci. 213, 36–45.
Pontoni D., T. Narayanan & A.R. Rennie, 2002. Time-resolved SAXS study of nucleation and growth of silica colloids. Langmuir 18, 56–59.
Sau T.K., A. Pal & T. Pal, 2001. Size regime dependent catalysis by gold nanoparticles for the reduction of eosin. J. Phys. Chem. B 105, 9266–9272.
Sloezynski J. & W. Bobinski, 1991. Autocatalytic effect in the processes of metal oxide. J. Solid State Chem. 92, 420–448.
Talapin D.V., A.L. Rogach, M. Hasse & H. Weller, 2001. Evolution of an ensemble of nanoparticles in a colloidal solution: Theoretical study. J. Phys. Chem. B 105, 12278–12285.
Treguer M., C. de Cointet, H. Remita, J. Khatouri, M. Mostafavi, J. Amblard, J. Belloni & R. de Keyzer, 1998. Dose rate effects on radiolytic synthesis of gold–silver bimetallic clusters in solution. J. Phys. Chem. B 102, 4310–4321.
Wang Y. & N. Herron, 1996. X-ray photoconductive nanocomposites. Science 273, 632–634.
Whetten R.L., M.N. Shafigullin, J.T. Khoury, T.G. Schaaff, I. Vezmar, M.M. Alvarez & A. Wilkinson, 1999. Crystal structures of molecular gold nanocrystal arrays. Acc. Chem. Res. 32, 397–406.
Yonezawa Y., T. Sato, S. Kuroda & K. Kuge, 1991. Photochemical formation of colloidal silver: Peptizing action of acetone ketyl radical. J. Chem. Soc. Faraday Trans. 87, 1905–1910.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar Ghosh, S., Kundu, S., Mandal, M. et al. Studies on the Evolution of Silver Nanoparticles in Micelle by UV-Photoactivation. Journal of Nanoparticle Research 5, 577–587 (2003). https://doi.org/10.1023/B:NANO.0000006100.25744.fa
Issue Date:
DOI: https://doi.org/10.1023/B:NANO.0000006100.25744.fa