Abstract
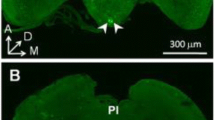
The role of serotonin in controlling feeding in flesh flies is examined. Amount of feeding was recorded over 6 h for flies injected with serotonin or saline. The proportion of time spent on various behaviors over a period of 1 h was recorded after the injection of serotonin or saline or no injection. Corresponding electrophysiological measurements were made on serotonin and saline-injected flies. The release of serotonin as a consequence of feeding was also examined. The subesophageal ganglia of flies taken before or after 2 days of sugar feeding were examined immunocytochemically. Serotonin injection decreased feeding in flies compared to saline-injected flies. All behaviors measured decreased after serotonin injection, except for resting, which increased, and grooming, which decreased in both serotonin- and saline-injected flies. A marked reduction in electrophysiological responses to sucrose was seen in serotonin-injected flies. Specific cells of the subesophageal ganglion showed significantly less serotonin immunoreactivity in fed flies compared to flies that had not yet fed. The role of serotonin in affecting the physiology of feeding in insects is discussed.
Similar content being viewed by others
References
Albert, P. J., Smith, J. J. B., Mitchell, B. K., and Whitehead, A. T. (1991). Differences in response patterns of labellar chemosensilla of the flesh fly Sarcophaga bullata. Comp. Biochem. Physiol. 100A: 681–687.
Ali, D. W. (1997). The aminergic and peptidergic innervation of insect salivary glands. J. Exp. Biol. 200: 1941–1947.
Angioy, A. M., Liscia, A., and Pietra, P. (1980). Electrical resistance and spike activity in tarsal chemosensilla of Phormia regina (Meig.). Experientia 36: 962.
Angioy, A. M., Liscia, A., and Pietra, P. (1979). Electrical resistance of labellar taste hairs in the blowfly, Phormia regina M., as a function of age and sex. Boll. Soc. Ital. Biol. Speriment. 55: 2100–2104.
Bellamy, F. W., and Zacharuk, R. Y. (1976). Structure of the labial palp of a larval elaterid and of the sinus cells associated with its sensilla. Can. J. Zool. 54: 2118–2128.
Bicker, G., and Menzel, R. (1989). Chemical codes for the control of the behavior in arthropods. Nature 337 (6202): 33–44
Bishop, C. A., and O'Shea, M. (1982). Serotonergic immunoreactive neurons in the central nervous system of an insect (Periplaneta americana). J. Neurobiol. 14(4): 251–269.
Blaney, W. M., Schoonhoven, L. M., and Simmonds, M. S. J. (1986). Sensitivity variations in insect chemoreceptors; A review. Experientia 42: 13–19.
Cantera, R., and Nässel, D. R. (1987). Postembryonic development of serotonin-immunoreactive neurons in the central nervous system of the blowfly. Cell Tissue Res. 250: 449–459.
Davis, N. T. (1987). Neurosecretory neurons and their projections to the serotonin neurohaemal system of the cockroach Periplaneta americana (L.) and identification of mandibular and maxillary motor neurons associated this system. J. Comp. Neurol. 259: 604–621.
Dethier, V. G. (1976). The Hungry Fly, Harvard University Press, Cambridge, MA
Dolzer, J., Krannich, S., Fischer, K., and Stengl, M. (2001). Oscillations of the transeptithelial potential of moth olfactory sensilla are influenced by octopamine and serotonin. J. Exp. Biol. 204: 2781–2794.
Gnatzy, W., and Weber, K. M. (1978). Tormogen cell and receptor-lymph space in insect olfactory sensilla. Fine structure and histochemical properties in Calliphora. Cell Tissue Res. 189: 549–554.
Grostmaitre, X., Marion-Poll, F., and Renou, M. (2001). Biogenic amines modulate olfactory receptor neurons firing activity in Mamestra brassicae. Chem. Senses 26: 653–661.
Helles, J., Dircksen, H., Eckert, M., Nässel, D. R., Spoerhase-Eichmann U., and Schuermann, F. (1995). Putative neurohemal areas in the peripheral nervous system of an insect, Gryllus bimaculatus, revealed by immunocytochemistry. Cell Tissue Res. 281(1): 43–61.
Homberg, U., and Hildebrand, J. G. (1989). Serotonin immunoreactive neurons in the median protocerebrum and subsesophageal ganglion of the sphinx moth Manduca sexta. Cell Tissue Res. 258: 1–24.
Hörner, M., Spörhase-Eichmann, U., Helle, J., Venus, B., and Schürmann, F. W. (1995). The distribution of neurones immunoreactive for β-tyrosine hydroxylase, dopamine and serotonin in the ventral nerve cord of the cricket, Gryllus bimaculatus. Cell Tissue Res. 280: 583–604.
Kent, K. S., Hoskins, S. G., and Hildebrand, J. G. (1987). A novel serotonin-immunoreactive neuron in the antennal lobe of the sphinx moth Manduca sexta persists throughout postembryonic development. J. Neurobiol. 18(5): 451–465.
Kijima, H., Okada, Y., Oiki, S., Goshima, S., Nagata, K., and Kazawa, T. (1995). Free ion concentrations in receptor lymph and role of transepithelial voltage in the fly labellar taste receptor. J. Comp. Physiol. A 177(2): 123–133.
Kloppenburg, P., Ferns, D., and Mercer, A. (1999). Serotonin enhances central olfactory neuron responses to female sex pheromone in the male sphinx moth Manduca sexta. J. Neurosci. 19(19: 8172–8181.
Kloppenburg, P., and Hildebrand, J. G. (1995). Neuromodulation by 5–hydroxytryptamine in the antennal lobe of the sphinx moth Manduca sexta. J. Exp. Biol. 198: 603–611.
Kloppenburg, P., and Heinbockel, T. (2000). 5–Hydroxytryptamine modulates pheromone-evoked local field potentials in the macroglomerular complex of the sphinx moth Manduca sexta. J. Exp. Biol. 203: 1701–1709
Küppers, J., and Thurm, U. (1975). Humorales steuerung eines ionentransports an epithelialen rezeptoern von insekten. Verh. Dt. Zool. Ges. 67: 46–50.
Küppers, J., and Thurm, U. (1982). On the functional significance of ion circulation induced by electrogenic transport. In Addink, A. D. F., and Spronk, N. (Eds.), Exogenous and Endogenous Influences on Metabolic and Neural Control, Pergamon Press, Oxford, Vol. 1, pp. 313–327.
Lange, A. B., Orchard, I., and Lloyd, R. J. (1988). Immunohistochemical and electrochemical detection of serotonin in the nervous system of the blood-feeding bug, Rhodnius prolixus. Arch. Insect Biochem. Physiol. 8: 187–201.
Lange, A. B., Orchard, I., and Barrett, F. M. (1989). Changes in the haemolymph serotonin levels associated with feeding in the blood-sucking bug, Rhodnius prolixus. J. Insect Physiol. 35: 393–399.
Leibowitz, S. F., and Alexander, J. T. (1998). Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiat. 44: 851–864.
Leibowitz, S. F., Weiss, G. F., and Shor-Posner, G. (1988). Hypothalamic serotonin: Pharmacological, biochemical, and behavioral analyses of its feeding-suppressive action. Clin. Neuropharmacol. 11: 51–71.
Liscia, A., Majone, R., Solari, P, Tomassini Barbarossa, I., and Crnjar, R. (1998). Sugar response differeces related to sensillum type and location of the labella of Protophormia terraenovae: A contribution to spatial representation of the stimulus. J. Insect Physiol. 44: 471–481.
Long, T. F., Edgecomb, R. S., and Murdock, L. L. (1986). Effects of phenylethylamines on blowfly feeding behavior. Comp. Biochem. Physiol. C 83(1): 201–209.
Long, T. F., and Murdock, L. (1983). Stimulation of blowfly feeding behavior by octopaminergic drugs. Proc. Natl. Acad. Sci. USA 80: 4159.
Longley, A. J., and Longley, R. D. (1986). Serotonin immunoreactivity in the nervous system of the dragonfly nymph. J. Neurobiol. 17: 329–338.
Mason, S. T. (1984). Catecholamines and Behaviour, Cambridge University Press, London.
Mercer, A. R., Hayashi, J. H., and Hildebrand, J. G. (1995). Modulatory effects of 5–hydroxytryptamine on voltage-activated currents in cultured antennal lobe neurons of the sphinx moth Manduca sexta. J. Exp. Biol. 198: 613–627.
Nässel, D. R. (1987). Serotonin and serotonin-immunoreactive neurons in the nervous system of insects. Prog. Neurobiol. 30: 1–85.
Nässel, D. R., and Cantera, R. (1985). Mapping of serotonin-immunoreactive neurons in the larval nervous system of the flies Calliphora erythrocephala and Sarcophaga bullata. Cell Tissue Res. 239: 423–434.
Nässel, D. R., and Elekes, K. (1985). Serotonergic terminals in the neural sheath of the blowfly nervous system: Electron microscopical immunocytochemistry and 5,7–dihydroxytryptamine labeling. Neuroscience 15(1): 293–307.
Phillips, C. E., and Vande Berg, J. S. (1976). Mechanism for sensillum fluid flow in trichogen and tormogen cells of Phormia regina (Meigen). Int. J. Insect Morphol. Embryol. 5: 423–431.
Pietra, P., Angoiy, A. M., Liscia, A., and Crnjar, R. (1978). Electrical resistivity of labellar taste hairs of male and female blowflies, Phormia regina (Meig.). Experientia 34: 491.
Pietra, P., Angoiy, A. M., Liscia, A., and Crnjar, R. (1979a). Influence of the viscous substance at the tip of the labellar hairs of Phormia regina M. on the effectiveness of stimulation by cations and anions. Experientia 35: 1195–1196.
Pietra, P., Angoiy, A. M., Liscia, A., and Crnjar, R. (1979b). Importance of the viscous substance at the tip of the labellar taste hairs of Phormia regina M. on the salt-sugar interaction. Experientia 35: 1196.
Pietra, P., Angoiy, A. M., Liscia, A., and Crnjar, R. (1980a). Electrical resistance and spike activity in tarsal chemosensilla of Phormia regina (Meig.) Experientia 36: 962
Pietra, P., Sigiru, P., Angioy, A. M., and Liscia, A. (1980b). The presence of acid mucopolysaccharides in viscous extrusions from the labellar and tarsal chemosensilla of Phormia regina (Meig.). Basic Appl. Histochem. 24: 53–57.
Pophof, B. (2000). Octopamine modulates the sensitivity of silkmoth pheromone receptor neurons. J. Comp. Physiol. A 186: 307–313.
Schachtner, J., and Braünig, P. (1993). The activity patterns of identified neurosecretory cells during feeding behaviour in the locust. J. Exp. Biol. 185: 287–303.
Schnuch, M., and Seebauer, H. (1998). Sugar cell responses to lactose and sucrose in labellar and tarsal taste hairs of Musca domestica. J. Comp. Physiol. A 182: 767–775.
Schulz, D., and Robinson, G. (2001). Octopamine influences division of labor in honey bees. J. Comp. Physiol. A 187: 53–61.
Scott, D. A., and Zacharuk, R. Y. (1971). Fine structure of the dendritic junction body region of the antennal sensory cone in a larval elaterid. Can. J. Zool. 49: 817–821.
Shor-Posner, G., Grinker, J. A., Marinescu, C., Brown, O., and Leibowitz, S. F. (1986). Hypothalamic serotonin in the control of meal patterns and macronutrient selection. Brain Res. Bull. 17: 663–671.
Simpson, S. J., and Raubenheimer, D. (1995). The geometric analysis of feeding and nutrition: A user's guide. J. Insect Physiol. 41(7): 545–553.
Simpson, S. J., Browne, L. B., and van Gerwen, A. C. M. (1989). The patterning of compensatory sugar feeding in the Australian sheep blowfly. Physiol. Entomol. 14: 91–105.
Smith, J. J. B., Mitchell, B. K., Rolseth, B. M., Whitehead, A. T., and Albert, P. J. (1991). SAPID Tools: Micro-computer programs for analysis of multi-unit nerve recordings. Chem. Senses 15: 253–270.
Sudlow, L. C., Edgecomb, R. S., and Murdock, L. L. (1987). Regulation of labellar and tarsal taste thresholds in the black blowfly, Phormia regina. J. Exp. Biol. 130: 219–234.
Trimmer, B. A. (1985a). The inactivation of exogenous serotonin in the blowfly, Calliphora. Insect Biochem. 15(4: 435–442.
Trimmer, B. A. (1985b). Serotonin and the control of salivation in the blowfly Calliphora. J. Exp. Biol. 114: 307–328.
Tsang, P. W., and Orchard, I. (1991). Distribution of FMRFamide-related peptides in the blood-feeding bug, Rhodnius prolixus. J. Comp. Neurol. 311: 17–32.
Tyrer, N. M., Turner, J. D., and Altman, J. S. (1984). Identifiable neurons in the locust central nervous system that react with antibodies to serotonin. J. Comp. Neurol. 227: 313–330.
Vallés, A. M., and White, K. (1988). Serotonin-containing in Drosophila melanogaster: Development and distribution. J. Comp. Neurol. 268: 414–428.
Van Der Wolk, F. M., Koerten, H. K., and Van Der Starre, H. (1984). The external morphology of contact-chemoreceptive hairs of flies and the motility of the tips of these hairs. J. Morphol. 180: 37–54.
Weiss, G. F., Papadakos, P., Knudson, K., and Leibowitz, S. F. (1986). Medial hypothalamic serotonin: Effects on deprivation and norepinephrine-induced eating. Pharmocol. Biochem. Behav. 25: 1223–1230.
Weiss, G. F., Rogacki, N., Fueg, A., Buchen, D., and Leibowitz, S. F. (1990). Impact of hypothalamic d-norfenfluramine and peripheral fluoxetine injection on macronutrient intake in the rat. Brain Res. Bull. 25: 849–859.
Weiss, G. F., Rogacki, N., Fueg, A., et al. (1991). Effect of hypothalamic and peripheral fluoxetine injection on natural patterns of macronutrient intake in the rat. Psychopharmacology 105: 467–476.
Wilczek, M. (1967). The distribution and neuroanatomy of the labellar sense organs of the blowfly, Phormia regina Meigen. J. Morphol. 122: 175–201.
Würden, S., and Homberg, U. (1995). Immunocytochemical mapping of serotonin and neuropeptides in the accessory medulla of the locust Schistocerca gregaria. J. Comp. Neurol. 362: 305–319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dacks, A.M., Nickel, T. & Mitchell, B.K. An Examination of Serotonin and Feeding in the Flesh Fly Neobellieria bullata (Sarcophagidae: Diptera). Journal of Insect Behavior 16, 1–21 (2003). https://doi.org/10.1023/A:1022817610378
Issue Date:
DOI: https://doi.org/10.1023/A:1022817610378