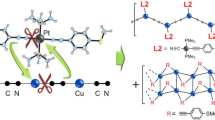
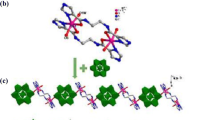
Abstract
Organic ligand-based transition metal coordinated complexes have been of paramount importance. They have found a lot of utilities in catalysis, bond activation, dye-sensitized solar cells, etc. Focusing on the so-called noble metal or coinage metal and keeping in mind the inexpensive single metal atom based catalyst, copper bound complexes are considered for adsorption of gas molecules and bond activation therein. Here, we have studied the interaction between a [Cu–NN] complex (N,N-coordinated mono-cationic copper bound Pyrazol-1-yl(1H-pyrrol-2-yl)methanone) and different gas molecules as ligands, such as L = H2, N2, CO, H2O, C2H4 and C2H2, which are industrially and environmentally important. Our computational investigation infers that these ligands are effective in electronic interactions and optical properties of the complex. The stability, geometry and the bonding nature in L bound [Cu–NN] complexes are studied to check their viability at room temperature. The [Cu–NN] complex can bind small gas molecules as ligands, viz., H2, N2, CO, H2O, C2H4 and C2H2, in a thermodynamically favorable way and the complexation induces bond activation within the ligands in the bound state as compared to their free state. All the [L–Cu–NN] complexes along with the bare complex show broadband optical absorption in the ultraviolet–visible (UV–Vis) domains. Furthermore, selective ligand binding has modulated the Fermi energy level resulting in an enhanced chemical reactivity of the [Cu–NN] complex.




Similar content being viewed by others
References
Bader, R.F.: The characterization of atomic interactions. J. Chem. Phys. 80(5), 1943–1960 (1984)
Bai, S.L.: High-efficiency direct methane conversion to oxygenates on a cerium dioxide nanowires supported rhodium single-atom catalyst. Nat. Commun. 11(1), 1–9 (2020)
Baronia, R.G.: Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrog. Energy 42(15), 10238–10247 (2017)
Boys, S.F.: The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19(4), 553–566 (1970)
Brown, T.J.: Syntheses, X-ray crystal structures, and solution behavior of monomeric, cationic, two-coordinate Gold (I) π-alkene complexes. J. Am. Chem. Soc. 131(18), 6350–6351 (2009)
Bunting, R.J.: The mechanism and ligand effects of single atom rhodium supported on ZSM-5 for the selective oxidation of methane to methanol. Phys. Chem. Chem. Phys. 22(20), 11686–11694 (2020)
Calderón Gómez, J.C.: Palladium-based catalysts as electrodes for direct methanol fuel cells: a last ten years review. Catalysts 6(9), 130 (2016)
Chelucci, G.B.: Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions. Acc. Chem. Res. 48(2), 363–379 (2015)
Chen, X.S.: Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110(11), 6503–6570 (2010)
Chirik, P.J.: In: Mingos, D.M.P. (ed.) Comprehensive Organometallic Chemistry III. Elsevier, Oxford (2007)
Cinellu, M.A.: Reaction of gold (iii) oxo complexes with alkenes. Synthesis of unprecedented gold alkene complexes,[Au (N, N)(alkene)][PF 6]. Crystal structure of [Au (bipy ip)(η 2-CH 2 [double bond, length as m-dash] CHPh)][PF 6](bipy ip= 6-isopropyl-2, 2′-bipyridine). Chem. Commun. 14, 1618–1619 (2004)
Cinellu, M.A.: Synthesis and properties of gold alkene complexes. Crystal structure of [Au (bipy oXyl)(η 2-CH 2 [double bond, length as m-dash] CHPh)](PF 6) and DFT calculations on the model cation [Au (bipy)(η 2-CH 2 [double bond, length as m-dash] CH 2)]+. Dalton Trans. 48, 5703–5716 (2006)
Coolidge, A.S.: A study of the Franck-Condon principle. J. Chem. Phys. 4(3), 193–211 (1936)
Cremer, D.: Chemical bonds without bonding electron density. Angew Chem Int Ed Engl. 23, 627–628 (1984)
D.F. MJ Frisch, G. T.: Gaussian D~0 (2014)
Dias, H.R.: Thermally stable Gold (I) ethylene adducts: [(HB 3, 5-(CF3) 2Pz3) Au (CH2=CH2)] and [(HB3-(CF3), 5-(Ph) Pz3) Au (CH2=CH2)]. Angew. Chem. Int. Ed. 46(41), 7814–7816 (2007)
Dias, H.R.: Synthesis and characterization of the gold (I) tris (ethylene) complex [Au (C2H4)3][SbF6]. Angew. Chem. 120(3), 566–569 (2008)
Dias, H.R.: Monomeric copper (I), silver (I), and gold (I) alkyne complexes and the coinage metal family group trends. J. Am. Chem. Soc. 131(31), 11249–11255 (2009)
Dunning, T.H., Jr.: Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90(2), 1007–1023 (1989)
Eren, B.Z.: Activation of Cu (111) surface by decomposition into nanoclusters driven by CO adsorption. Science 351(6272), 475–478 (2016)
Ertl, G.: Reactions at surfaces: from atoms to complexity (Nobel lecture). Angew. Chem. Int. Ed. 47(19), 3524–3535 (2008)
Feary, J. (2019). Heavy Metals Reference Module in Biomedical Sciences [Internet]. Elsevier, Amsterdam
Galletti, C.S.: Catalytic performance of rhodium-based catalysts for CO preferential oxidation in H2-rich gases. Ind. Eng. Chem. Res. 47(15), 5304–5312 (2008)
Gao, S.Y.: One-step synthesis of Pt based electrocatalysts encapsulated by polyoxometalate for methanol oxidation. New J. Chem. 42(1), 198–203 (2018)
Glendening, E.D.: NBO 6.0: natural bond orbital analysis program. J. Comput. Chem. 34(16), 1429–1437 (2013)
Haakansson, M.J.: A complex between isoprene and copper (I) chloride: synthesis and structural characterization. Organometallics 10(5), 1317–1319 (1991)
Hisatomi, T.K.: Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43(22), 7520–7535 (2014)
Hölscher, M.: Frontispiece: catalytic NH3 synthesis using N2/H2 at molecular transition metal complexes: concepts for lead structure determination using computational chemistry. Chem.: Eur. J. 23(50), 11992–12003 (2017)
Hooper, T.N.: Synthesis and structural characterisation of stable cationic gold (I) alkene complexes. Chem. Commun. 26, 3877–3879 (2009)
Hülsey, M.J.: In situ spectroscopy-guided engineering of rhodium single-atom catalysts for CO oxidation. Nat. Commun. 10(1), 1–10 (2019)
Huzinaga, S.: A comparison of the geometrical sequence formula and the well-tempered formulas for generating GTO basis orbital exponents. Chem. Phys. Lett. 175(4), 289–291 (1990)
Jana, G.S.: Noble gas binding ability of metal-bipyridine monocationic complexes (metal= Cu, Ag, Au): a computational study. ChemistrySelect 1(18), 5842–5849 (2016)
Kaneti, J.D.: Computational probes into the basis of silver ion chromatography. II. Silver (I)—olefin complexes. J. Phys. Chem. A 106(46), 11197–11204 (2002)
Kwon, Y.K.: Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. J. Am. Chem. Soc. 139(48), 17694–17699 (2017)
Liu, J.S.-S.: Palladium–gold single atom alloy catalysts for liquid phase selective hydrogenation of 1-hexyne. Catal. Sci. Technol. 7(19), 4276–4284 (2017)
Lu, T.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012)
Macchi, P.P.: Experimental electron density studies for investigating the metal π-ligand bond: the case of bis (1, 5-cyclooctadiene) nickel. J. Am. Chem. Soc. 120(7), 1447–1455 (1998)
Macchi, P.G.: Charge density in transition metal clusters: supported vs unsupported metal-metal interactions, pp. 10428–10429 (1999)
Medvedev, M.G.: Density functional theory is straying from the path toward the exact functional. Science 355(6320), 49–52 (2017a)
Medvedev, M.G.: Response to comment on “Density functional theory is straying from the path toward the exact functional.” Science 356(6337), 496–496 (2017b)
Musashi, Y.: Theoretical study of rhodium (III)-catalyzed hydrogenation of carbon dioxide into formic acid. Significant differences in reactivity among rhodium (III), rhodium (I), and ruthenium (II) complexes. J. Am. Chem. Soc. 124(25), 7588–7603 (2002)
Park, M.J.: Enhanced chemical reactivity of graphene by Fermi level modulation. Chem. Mater. 30(16), 5602–5609 (2018)
Perdew, J.P.: Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105(22), 9982–9985 (1996)
Peterson, K.A.: Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H+ H2→ H2+ H reaction. J. Chem. Phys. 100(10), 7410–7415 (1994)
Peterson, K.A.: Systematically convergent basis sets for transition metals. II. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor. Chem. Acc. 114(4), 283–296 (2005)
Reed, A.E.: Natural bond orbital analysis of near‐Hartree–Fock water dimer. J. Chem. Phys. F 8, 4066 (1983)
Reed, A.E.: Natural population analysis. J. Chem. Phys. 83(2), 735–746 (1985)
Reiss, H.: The Fermi level and the redox potential. J. Phys. Chem. 89(18), 3783–3791 (1985)
Saranya, S.: Copper Catalysis: An Introduction. Copper Catalysis in Organic Synthesis. Wiley Online Library, Hoboken (2020)
Schaller, G.E.: Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270(5243), 1809–1811 (1995)
Schmidbaur, H.: Noble Metals (Chemistry). Elsevier, Amsterdam (2003)
Su, N.Q.: Doubly hybrid density functionals that correctly describe both density and energy for atoms. Proc. Natl. Acad. Sci. 115(10), 2287–2292 (2018)
Tang, J.D.: Rhodium catalysts with cofactor mimics for the biomimetic reduction of C [double bond, length as m-dash] N bonds. Catal. Sci. Technol. 11(16), 5564–5569 (2021)
Te Velde, G.T., Bickelhaupt, F.M., Baerends, E.J., Fonseca Guerra, C., van Gisbergen, S.J.: Chemistry with ADF. J. Comput. Chem. 22(9), 931–967 (2001)
Ukai, K.A.: Rhodium (I)-catalyzed carboxylation of aryl-and alkenylboronic esters with CO2. J. Am. Chem. Soc. 128(27), 8706–8707 (2006)
Ullrich, C.A.: A brief compendium of time-dependent density functional theory. Braz. J. Phys. 44(1), 154–188 (2014)
Wiberg, K.B.: Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24(3), 1083–1096 (1968)
Woon, D.E.: Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties. J. Chem. Phys 100, 2975–2988 (1994)
Wu, L.L.: Highly regioselective osmium-catalyzed hydroformylation. Chem. Commun. 51(15), 3080–3082 (2015)
Xiao, M.Z.: A single-atom iridium heterogeneous catalyst in oxygen reduction reaction. Angew. Chem. Int. Ed. 58(28), 9640–9645 (2019)
Yao, Z.J.: [NO]-and [NN]-coordination mode rhodium complexes based on a flexible ligand: synthesis, reactivity and catalytic activity. New J. Chem. 40(10), 8753–8759 (2016)
Zhao, Y.Y.: Stable iridium dinuclear heterogeneous catalysts supported on metal-oxide substrate for solar water oxidation. Proc. Natl. Acad. Sci. 115(12), 2902 (2018a)
Zhao, L.V.: Energy decomposition analysis. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 8(3), e1345 (2018b)
Zimmermann, S.: Significance of platinum group metals emitted from automobile exhaust gas converters for the biosphere. Environ. Sci. Pollut. Res. 11(3), 194–199 (2004)
Acknowledgements
PKC thanks Professor Chandrima Saha, President, INSA, for kindly inviting him to contribute an article to this journal as a part of his Professor Sadhan Basu Memorial Lecture. PKC would also like to thank DST, New Delhi for the J. C. Bose National Fellowship. RJ and GJ acknowledge Indian Institute of Technology Kharagpur and Indian Institute of Technology Bombay, respectively, for their Research Fellowships.
Author information
Authors and Affiliations
Contributions
RJ: Methodology, Software, Writing—first draft. GJ: Data analysis, Critical scrutiny and editing of original Manuscript, Investigation, PKC: Supervision, Investigation, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jha, R., Jana, G. & Chattaraj, P.K. Possible catalytic activity of N,N-coordinated mono-cationic copper bound Pyrazol-1-yl(1H-pyrrol-2-yl)methanone complex: a computational study. Proc.Indian Natl. Sci. Acad. 88, 172–185 (2022). https://doi.org/10.1007/s43538-022-00072-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43538-022-00072-7