Abstract
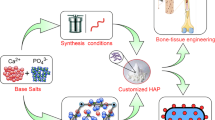
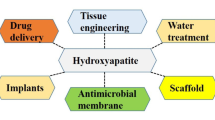
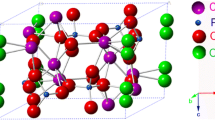
Hydroxyapatite (HA) is one of the most common bioceramics and is abundant in human bones. HA is composed of calcium phosphate, which is prevalent in biomedical processes, particularly bone formation, osteogenesis, and angiogenesis. As HA is one of the core materials that makes up the human body, there has been considerable research on methods of synthesizing HA while changing its properties by substituting various types of metal ions. In particular, previous studies have intensively investigated the size, crystallinities, and morphologies generated using various synthesis methods to change the characteristics of HA by substituting different metal ions. This review summarizes the findings of these studies on HA, including findings on the characteristics of HA in natural bone, methods of synthesizing HA, and findings on metal-ion-substituted HA. Furthermore, the characteristics and applications of HA that were investigated in previous studies are summarized, and the latest trends and perspectives on the future of the field are also presented.







Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Z. Tang, Y. Tan, Y. Ni, J. Wang, X. Zhu, Y. Fan, X. Chen, X. Yang, X. Zhang, Comparison of ectopic bone formation process induced by four calcium phosphate ceramics in mice. Mater. Sci. Eng. C 70, 1000–1010 (2017). https://doi.org/10.1016/j.msec.2016.06.097
Y. Deng, Y. Yang, Y. Ma, K. Fan, W. Yang, G. Yin, Nano-hydroxyapatite reinforced polyphenylene sulfide biocomposite with superior cytocompatibility and in vivo osteogenesis as a novel orthopedic implant. RSC Adv. 7, 559–573 (2017). https://doi.org/10.1039/c6ra25526d
J.H. Lee, G.S. Yi, J.W. Lee, D.J. Kim, Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant Sci. 47, 388–401 (2017). https://doi.org/10.5051/jpis.2017.47.6.388
T.H. Lim, J.R. Choi, D.Y. Lim, S.H. Lee, S.Y. Yeo, Preparation of fiber based binder materials to enhance the gas adsorption efficiency of carbon air filter. J. Nanosci. Nanotechnol. 15, 8034–8041 (2015). https://doi.org/10.1166/jnn.2015.11276
L.F. Sukhodub, L.B. Sukhodub, A.D. Pogrebnjak, A. Turlybekuly, A. Kistaubayeva, I. Savitskaya, D. Shokatayeva, Effect of magnetic particles adding into nanostructured hydroxyapatite–alginate composites for orthopedics. J. Korean Ceram. Soc. 57, 557–569 (2020). https://doi.org/10.1007/s43207-020-00061-w
B. Mohapatra, T.R. Rautray, Strontium-substituted biphasic calcium phosphate scaffold for orthopedic applications. J. Korean Ceram. Soc. 57, 392–400 (2020). https://doi.org/10.1007/s43207-020-00028-x
C. Shuai, S. Li, S. Peng, P. Feng, Y. Lai, C. Gao, Biodegradable metallic bone implants. Mater. Chem. Front. 3, 544–562 (2019). https://doi.org/10.1039/c8qm00507a
N. Strutynska, A. Malyshenko, N. Tverdokhleb, M. Evstigneev, L. Vovchenko, Y. Prylutskyy, N. Slobodyanik, U. Ritter, Design, characterization and mechanical properties of new Na+, CO32−-apatite/alginate/C60 fullerene hybrid biocomposites. J. Korean Ceram. Soc. 58, 422–429 (2021). https://doi.org/10.1007/s43207-020-00107-z
J.W. Lee, S. Chae, S. Oh, S.H. Kim, K.H. Choi, M. Meeseepong, J. Chang, N. Kim, Y.H. Kim, N.E. Lee, J.H. Lee, J.Y. Choi, Single-chain atomic crystals as extracellular matrix-mimicking material with exceptional biocompatibility and bioactivity. Nano Lett. 18, 7619–7627 (2018). https://doi.org/10.1021/acs.nanolett.8b03201
E. Saygili, E. Kaya, E. Ilhan-Ayisigi, P. Saglam-Metiner, E. Alarcin, A. Kazan, E. Girgic, Y.W. Kim, K. Gunes, G.G. Eren-Ozcan, D. Akakin, J.Y. Sun, O. Yesil-Celiktas, An alginate-poly(acrylamide) hydrogel with TGF-β3 loaded nanoparticles for cartilage repair: biodegradability, biocompatibility and protein adsorption. Int. J. Biol. Macromol. 172, 381–393 (2021). https://doi.org/10.1016/j.ijbiomac.2021.01.069
J.H. Lee, I.H. Ko, S.H. Jeon, J.H. Chae, J.H. Chang, Micro-structured hydroxyapatite microspheres for local delivery of alendronate and BMP-2 carriers. Mater. Lett. 105, 136–139 (2013). https://doi.org/10.1016/j.matlet.2013.04.082
H.G. Jung, D. Lee, S.W. Lee, I. Kim, Y. Kim, J.W. Jang, J.H. Lee, G. Lee, D.S. Yoon, Nanoindentation for monitoring the time-variant mechanical strength of drug-loaded collagen hydrogel regulated by hydroxyapatite nanoparticles. ACS Omega 6, 9269–9278 (2021). https://doi.org/10.1021/acsomega.1c00824
M. Park, J.C. Pyun, J. Jose, Orientation and density control of proteins on solid matters by outer membrane coating: analytical and diagnostic applications. J. Pharm. Biomed. Anal. 147, 174–184 (2018). https://doi.org/10.1016/j.jpba.2017.07.043
J.H. Kim, S.H. Kim, H.K. Kim, T. Akaike, S.C. Kim, Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods. J. Biomed. Mater. Res. 62, 600–612 (2002). https://doi.org/10.1002/jbm.10280
B. Cengiz, Y. Gokce, N. Yildiz, Z. Aktas, A. Calimli, Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 322, 29–33 (2008). https://doi.org/10.1016/j.colsurfa.2008.02.011
G.B.C. Cardoso, A. Tondon, L.R.B. Maia, M.R. Cunha, C.A.C. Zavaglia, R.R. Kaunas, In vivo approach of calcium deficient hydroxyapatite filler as bone induction factor. Mater. Sci. Eng. C 99, 999–1006 (2019). https://doi.org/10.1016/j.msec.2019.02.060
R.M.G. Rajapakse, W.P.S.L. Wijesinghe, M.M.M.G.P.G. Mantilaka, K.G. Chathuranga Senarathna, H.M.T.U. Herath, T.N. Premachandra, C.S.K. Ranasinghe, R.P.V.J. Rajapakse, M. Edirisinghe, S. Mahalingam, I.M.C.C.D. Bandara, S. Singh, Preparation of bone-implants by coating hydroxyapatite nanoparticles on self-formed titanium dioxide thin-layers on titanium metal surfaces. Mater. Sci. Eng. C 63, 172–184 (2016). https://doi.org/10.1016/j.msec.2016.02.053
L. Chen, Z. Wu, Y. Zhou, L. Li, Y. Wang, Z. Wang, Y. Chen, P. Zhang, Biomimetic porous collagen/hydroxyapatite scaffold for bone tissue engineering. J. Appl. Polym. Sci. 134, 1–8 (2017). https://doi.org/10.1002/app.45271
S.M. Lee, H.J. Byeon, B.H. Kim, J. Lee, J.Y. Jeong, J.H. Lee, J.H. Moon, C. Park, H. Choi, S.H. Lee, K.H. Lee, Flexible and implantable capacitive microelectrode for bio-potential acquisition. Biochip J. 11, 153–163 (2017). https://doi.org/10.1007/s13206-017-1304-y
E. Boanini, M. Gazzano, A. Bigi, Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 6, 1882–1894 (2010). https://doi.org/10.1016/j.actbio.2009.12.041
K. Sato, Mechanism of hydroxyapatite mineralization in biological systems. J. Ceram. Soc. Japan 115, 124–130 (2007). https://doi.org/10.2109/jcersj.115.124
S.H. Han, J.U. Lee, K.M. Lee, Y.Z. Jin, H. Yun, G.H. Kim, J.H. Lee, Enhanced healing of rat calvarial defects with 3D printed calcium-deficient hydroxyapatite/collagen/bone morphogenetic protein 2 scaffolds. J. Mech. Behav. Biomed. Mater. 108, 103782 (2020). https://doi.org/10.1016/j.jmbbm.2020.103782
M. Vallet-Regí, J.M. González-Calbet, Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 32, 1–31 (2004). https://doi.org/10.1016/j.progsolidstchem.2004.07.001
M. Sadat-Shojai, M.T. Khorasani, E. Dinpanah-Khoshdargi, A. Jamshidi, Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 9, 7591–7621 (2013). https://doi.org/10.1016/j.actbio.2013.04.012
A. Szcześ, L. Hołysz, E. Chibowski, Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 249, 321–330 (2017). https://doi.org/10.1016/j.cis.2017.04.007
J. Vecstaudza, M. Gasik, J. Locs, Amorphous calcium phosphate materials: formation, structure and thermal behaviour. J. Eur. Ceram. Soc. 39, 1642–1649 (2019). https://doi.org/10.1016/j.jeurceramsoc.2018.11.003
J.M. Thomann, J.C. Voegel, P. Gramain, Kinetics of dissolution of calcium hydroxyapatite powder. III: pH and sample conditioning effects. Calcif Tissue Int. 46, 121–129 (1990). https://doi.org/10.1007/BF02556096
L. Pighinelli, M. Kucharska, Chitosan-hydroxyapatite composites. Carbohydr. Polym. 93, 256–262 (2013). https://doi.org/10.1016/j.carbpol.2012.06.004
D.W. Hutmacher, Scaffolds in tissue engineering bone and cartilage. Biomater. Silver Jubil. Compend. 21, 175–189 (2000). https://doi.org/10.1016/B978-008045154-1.50021-6
K. Rezwan, Q.Z. Chen, J.J. Blaker, A.R. Boccaccini, Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27, 3413–3431 (2006). https://doi.org/10.1016/j.biomaterials.2006.01.039
S.V. Dorozhkin, Calcium orthophosphates in nature, biology and medicine. Materials (Basel) 2, 399–498 (2009). https://doi.org/10.3390/ma2020399
S.V. Dorozhkin, Calcium orthophosphates. J. Mater. Sci. 42, 1061–1095 (2007). https://doi.org/10.1007/s10853-006-1467-8
L.C. Chow, Solubility of calcium phosphates. Monogr. Oral Sci. 18, 94–111 (2001). https://doi.org/10.1159/000061650
R.I. Martin, P.W. Brown, Mechanical properties of hydroxyapatite formed at physiological temperature. J. Mater. Sci. Mater. Med. 6, 138–143 (1995). https://doi.org/10.1007/BF00120289
A. Gortemaker, J.A. Jansen, NHJC, Critical reviews in oral biology & medicine: aging and bone. J. Dent. Res. 89, 1333–1348 (2010). https://doi.org/10.1177/0022034510377791
C.H. Turner, Bone strength: current concepts. Ann. N. Y. Acad. Sci. 1068, 429–446 (2006). https://doi.org/10.1196/annals.1346.039
S.H. Ralston, Bone structure and metabolism. Medicine (United Kingdom) 45, 560–564 (2017). https://doi.org/10.1016/j.mpmed.2017.06.008
K.A. Hing, S.M. Best, W. Bonfield, Characterization of porous hydroxyapatite. J. Mater. Sci. Mater. Med. 10, 135–145 (1999). https://doi.org/10.1023/A:1008929305897
A.E. Jakus, A.L. Rutz, R.N. Shah, Advancing the field of 3D biomaterial printing. Biomed. Mater. (2016). https://doi.org/10.1088/1748-6041/11/1/014102
A. Liu, G.H. Xue, M. Sun, H.F. Shao, C.Y. Ma, Q. Gao, Z.R. Gou, S.G. Yan, Y.M. Liu, Y. He, 3D printing surgical implants at the clinic: a experimental study on anterior cruciate ligament reconstruction. Sci. Rep. 6, 1–13 (2016). https://doi.org/10.1038/srep21704
K.A. Hing, B. Annaz, S. Saeed, P.A. Revell, T. Buckland, Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. Mater. Med. 16, 467–475 (2005). https://doi.org/10.1007/s10856-005-6988-1
C. Kim, J.W. Lee, J.H. Heo, C. Park, D.H. Kim, G.S. Yi, H.C. Kang, H.S. Jung, H. Shin, Lee JH Natural bone-mimicking nanopore-incorporated hydroxyapatite scaffolds for enhanced bone tissue regeneration. Biomater Res. 26, 7 (2022). https://doi.org/10.1186/s40824-022-00253-x
G. Tripathi, B. Basu, A porous hydroxyapatite scaffold for bone tissue engineering: physico-mechanical and biological evaluations. Ceram. Int. 38, 341–349 (2012). https://doi.org/10.1016/j.ceramint.2011.07.012
J. Chen, Z. Wang, Z. Wen, S. Yang, J. Wang, Q. Zhang, Controllable self-assembly of mesoporous hydroxyapatite. Colloids Surf. B Biointerfaces 127, 47–53 (2015). https://doi.org/10.1016/j.colsurfb.2014.12.055
F. He, Y. Yang, J. Ye, Tailoring the pore structure and property of porous biphasic calcium phosphate ceramics by NaCl additive. Ceram. Int. 42, 14679–14684 (2016). https://doi.org/10.1016/j.ceramint.2016.06.092
S. Yunoki, T. Ikoma, A. Monkawa, K. Ohta, M. Kikuchi, S. Sotome, K. Shinomiya, J. Tanaka, Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Mater. Lett. 60, 999–1002 (2006). https://doi.org/10.1016/j.matlet.2005.10.064
P. Habibovic, M.C. Kruyt, M.V. Juhl, S. Clyens, R. Martinetti, L. Dolcini, N. Theilgaard, C.A. Van Blitterswijk, Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 26, 1363–1370 (2008). https://doi.org/10.1002/jor.20648
J.R. Woodard, A.J. Hilldore, S.K. Lan, C.J. Park, A.W. Morgan, J.A.C. Eurell, S.G. Clark, M.B. Wheeler, R.D. Jamison, A.J. Wagoner Johnson, The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 28, 45–54 (2007). https://doi.org/10.1016/j.biomaterials.2006.08.021
J.A. Juhasz, S.M. Best, W. Bonfield, Preparation of novel bioactive nano-calcium phosphate-hydrogel composites. Sci. Technol. Adv. Mater. (2010). https://doi.org/10.1088/1468-6996/11/1/014103
C.S. Ciobanu, S.L. Iconaru, I. Pasuk, B.S. Vasile, A.R. Lupu, A. Hermenean, A. Dinischiotu, D. Predoi, Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 33, 1395–1402 (2013). https://doi.org/10.1016/j.msec.2012.12.042
F.J. O’Brien, Biomaterials & scaffolds for tissue engineering. Mater. Today 14, 88–95 (2011). https://doi.org/10.1016/S1369-7021(11)70058-X
J. Biggemann, P. Müller, D. Köllner, S. Simon, P. Hoffmann, P. Heik, J.H. Lee, T. Fey, Hierarchical surface texturing of hydroxyapatite ceramics: influence on the adhesive bonding strength of polymeric polycaprolactone. J. Funct. Biomater. (2020). https://doi.org/10.3390/JFB11040073
A. Jaafar, C. Hecker, P. Árki, Y. Joseph, Sol-gel derived hydroxyapatite coatings for titanium implants: a review. Bioengineering 7, 1–23 (2020). https://doi.org/10.3390/bioengineering7040127
L. Gritsch, M. Maqbool, V. Mouriño, F.E. Ciraldo, M. Cresswell, P.R. Jackson, C. Lovell, A.R. Boccaccini, Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: copper and strontium. J. Mater. Chem. B 7, 6109–6124 (2019). https://doi.org/10.1039/c9tb00897g
L.L. Hench, Bioceramics. J. Am. Ceram. Soc. 81, 1705–1728 (1998)
A.F. Ali, Z.A. Alrowaili, E.M. El-Giar, M.M. Ahmed, A.M. El-Kady, Novel green synthesis of hydroxyapatite uniform nanorods via microwave-hydrothermal route using licorice root extract as template. Ceram. Int. 47, 3928–3937 (2021). https://doi.org/10.1016/j.ceramint.2020.09.256
J. Indira, K.S. Malathi, Comparison of template mediated ultrasonic and microwave irradiation method on the synthesis of hydroxyapatite nanoparticles for biomedical applications. Mater. Today Proc. (2021). https://doi.org/10.1016/j.matpr.2021.03.028
J.S. Earl, D.J. Wood, S.J. Milne, Hydrothermal synthesis of hydroxyapatite. J. Phys. Conf. Ser. 26, 268–271 (2006). https://doi.org/10.1088/1742-6596/26/1/064
D.M. Liu, T. Troczynski, W.J. Tseng, Water-based sol-gel synthesis of hydroxyapatite: process development. Biomaterials 22, 1721–1730 (2001). https://doi.org/10.1016/S0142-9612(00)00332-X
S.H. Rhee, Synthesis of hydroxyapatite via mechanochemical treatment. Biomaterials 23, 1147–1152 (2002). https://doi.org/10.1016/S0142-9612(01)00229-0
Y.J. Wang, C.J. Di, K. Wei, S.H. Zhang, X.D. Wang, Surfactant-assisted synthesis of hydroxyapatite particles. Mater. Lett. 60, 3227–3231 (2006). https://doi.org/10.1016/j.matlet.2006.02.077
A. Yelten-Yilmaz, S. Yilmaz, Wet chemical precipitation synthesis of hydroxyapatite (HA) powders. Ceram. Int. 44, 9703–9710 (2018). https://doi.org/10.1016/j.ceramint.2018.02.201
S.J. Lee, Y.S. Yoon, M.H. Lee, N.S. Oh, Nanosized hydroxyapatite powder synthesized from eggshell and phosphoric acid. J. Nanosci. Nanotechnol. 7, 4061–4064 (2007). https://doi.org/10.1166/jnn.2007.067
A. Buekenhoudt, A. Kovalevsky, J. Luyten, F. Snijkers, in 1.11 - basic aspects in inorganic membrane preparation, ed. by Drioli E, (Elsevier, Oxford, 2010), pp. 217–252. https://doi.org/10.1016/B978-0-08-093250-7.00011-6
S. Pramanik, A.K. Agarwal, K.N. Rai, A. Garg, Development of high strength hydroxyapatite by solid-state-sintering process. Ceram. Int. 33, 419–426 (2007). https://doi.org/10.1016/j.ceramint.2005.10.025
M.H. Santos, M. de Oliveira, L.P.F. de Souza, H.S. Mansur, W.L. Vasconcelos, Synthesis control and characterization of hydroxyapatite prepared by wet precipitation process. Mater. Res. 7, 625–630 (2004). https://doi.org/10.1590/s1516-14392004000400017
D. Moreno, F. Vargas, J. Ruiz, M.E. López, Solid-state synthesis of alpha tricalcium phosphate for cements used in biomedical applications. Bol. la Soc. Esp. Ceram. y Vidr. 59, 193–200 (2020). https://doi.org/10.1016/j.bsecv.2019.11.004
K. Teshima, S.H. Lee, M. Sakurai, Y. Kameno, K. Yubuta, T. Suzuki, T. Shishido, M. Endo, S. Oishi, Well-formed one-dimensional hydroxyapatite crystals grown by an environmentally friendly flux method. Cryst. Growth Des. 9, 2937–2940 (2009). https://doi.org/10.1021/cg900159j
H.R. Javadinejad, R. Ebrahimi-Kahrizsangi, Thermal and kinetic study of hydroxyapatite formation by solid-state reaction. Int. J. Chem. Kinet. 53, 583–595 (2021). https://doi.org/10.1002/kin.21467
T. Rojac, M. Kosec, Mechanochemical synthesis of complex ceramic oxides. High-Energy Ball Milling (2010). https://doi.org/10.1533/9781845699444.2.113
R. Dorey, Routes to thick films. Ceram. Thick Film MEMS Microdevices (2012). https://doi.org/10.1016/b978-1-4377-7817-5.00002-x
F.C. Gennari, J.J. Andrade-Gamboa, A systematic approach to the synthesis, thermal stability and hydrogen storage properties of rare-earth borohydrides, Emerging materials for energy conversion and storage (Elsevier, Amsterdam, Netherlands, 2018), pp. 429–459. https://doi.org/10.1016/B978-0-12-813794-9.00013-2
N. Llorca-Isern, C. Artieda-Guzmán, Metal-based composite powders, Advances in powder metallurgy: Properties, processing and applications (Elsevier Inc, Sawston, Cambridge, United Kingdom, 2013), pp. 241–72. https://doi.org/10.1533/9780857098900.2.241
R.B. Schwarz, Introduction to the viewpoint set on: mechanical alloying. Scr. Mater. 34, 1–4 (1996). https://doi.org/10.1016/1359-6462(95)00463-7
T.D. Shen, C.C. Koch, T.L. McCormick, R.J. Nemanich, J.Y. Huang, J.G. Huang, The structure and property characteristics of amorphous/nanocrystalline silicon produced by ball milling. J. Mater. Res. 10, 139–148 (1995). https://doi.org/10.1557/JMR.1995.0139
K.C.B. Yeong, J. Wang, S.C. Ng, Mechanochemical synthesis of nanocrystalline hydroxyapatite from CaO and CaHPO4. Biomaterials 22, 2705–2712 (2001). https://doi.org/10.1016/S0142-9612(00)00257-X
M.H. Fathi, E.M. Zahrani, Fabrication and characterization of fluoridated hydroxyapatite nanopowders via mechanical alloying. J. Alloys Compd. 475, 408–414 (2009). https://doi.org/10.1016/j.jallcom.2008.07.058
T.K. Achar, A. Bose, P. Mal, Mechanochemical synthesis of small organic molecules. Beilstein J. Org. Chem. 13, 1907–1931 (2017). https://doi.org/10.3762/bjoc.13.186
C. Shu, W. Yanwei, L. Hong, P. Zhengzheng, Y. Kangde, Synthesis of carbonated hydroxyapatite nanofibers by mechanochemical methods. Ceram. Int. 31, 135–138 (2005). https://doi.org/10.1016/j.ceramint.2004.04.012
S. Dinda, A. Bhagavatam, H. Alrehaili, G.P. Dinda, Mechanochemical synthesis of nanocrystalline hydroxyapatite from Ca(H2PO4)2·H2O, CaO, Ca(OH)2, and P2O5 mixtures. Nanomaterials 10, 1–10 (2020). https://doi.org/10.3390/nano10112232
S. Eiden-Aßmann, M. Viertelhaus, A. Heiß, K.A. Hoetzer, J. Felsche, The influence of amino acids on the biomineralization of hydroxyapatite in gelatin. J. Inorg. Biochem. 91, 481–486 (2002). https://doi.org/10.1016/S0162-0134(02)00481-6
J. Zhan, Y.H. Tseng, J.C.C. Chan, C.Y. Mou, Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to- single-crystal transformation. Adv. Funct. Mater. 15, 2005–2010 (2005). https://doi.org/10.1002/adfm.200500274
D.W. Kim, I.S. Cho, J.Y. Kim, H.L. Jang, G.S. Han, H.S. Ryu, H. Shin, H.S. Jung, H. Kim, K.S. Hong, Simple large-scale synthesis of hydroxyapatite nanoparticles: In situ observation of crystallization process. Langmuir 26, 384–388 (2010). https://doi.org/10.1021/la902157z
S. Catros, F. Guillemot, E. Lebraud, C. Chanseau, S. Perez, R. Bareille, J. Amédée, J.C. Fricain, Physico-chemical and biological properties of a nano-hydroxyapatite powder synthesized at room temperature. Irbm 31, 226–233 (2010). https://doi.org/10.1016/j.irbm.2010.04.002
Y.H. Huang, Y.J. Shih, F.J. Cheng, Novel KMnO 4-modified iron oxide for effective arsenite removal. J. Hazard Mater. 198, 1–6 (2011). https://doi.org/10.1016/j.jhazmat.2011.10.010
E. Bouyer, F. Gitzhofer, M.I. Boulos, Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 11, 523–531 (2000). https://doi.org/10.1023/A:1008918110156
A. Afshar, M. Ghorbani, N. Ehsani, M.R. Saeri, C.C. Sorrell, Some important factors in the wet precipitation process of hydroxyapatite. Mater. Des. 24, 197–202 (2003). https://doi.org/10.1016/S0261-3069(03)00003-7
T. Matsumoto, K.I. Tamine, R. Kagawa, Y. Hamada, M. Okazaki, J. Takahashi, Different behavior of implanted hydroxyapatite depending on morphology, size and crystallinity. J. Ceram. Soc. Japan 114, 760–762 (2006). https://doi.org/10.2109/jcersj.114.760
I. Mobasherpour, M.S. Heshajin, A. Kazemzadeh, M. Zakeri, Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 430, 330–333 (2007). https://doi.org/10.1016/j.jallcom.2006.05.018
G. Gecim, S. Dönmez, E. Erkoc, Calcium deficient hydroxyapatite by precipitation: continuous process by vortex reactor and semi-batch synthesis. Ceram. Int. 47, 1917–1928 (2021). https://doi.org/10.1016/j.ceramint.2020.09.020
Y. Wang, X. Ren, X. Ma, W. Su, Y. Zhang, X. Sun, X. Li, Alginate-intervened hydrothermal synthesis of hydroxyapatite nanocrystals with nanopores. Cryst. Growth Des. 15, 1949–1956 (2015). https://doi.org/10.1021/acs.cgd.5b00113
Y. Qi, J. Shen, Q. Jiang, B. Jin, J. Chen, X. Zhang, The morphology control of hydroxyapatite microsphere at high pH values by hydrothermal method. Adv. Powder Technol. 26, 1041–1046 (2015). https://doi.org/10.1016/j.apt.2015.04.008
G. Zhang, J. Chen, S. Yang, Q. Yu, Z. Wang, Q. Zhang, Preparation of amino-acid-regulated hydroxyapatite particles by hydrothermal method. Mater. Lett. 65, 572–574 (2011). https://doi.org/10.1016/j.matlet.2010.10.078
F. Nagata, Y. Yamauchi, M. Tomita, K. Kato, Hydrothermal synthesis of hydroxyapatite nanoparticles and their protein adsorption behavior. J. Ceram. Soc. Japan 121, 797–801 (2013). https://doi.org/10.2109/jcersj2.121.797
N.A.S. Mohd Pu’ad, R.H. Abdul Haq, H. Mohd Noh, H.Z. Abdullah, M.I. Idris, T.C. Lee, Synthesis method of hydroxyapatite: a review. Mater. Today Proc. 29, 233–239 (2019). https://doi.org/10.1016/j.matpr.2020.05.536
W.J. Shih, M.C. Wang, M.H. Hon, Morphology and crystallinity of the nanosized hydroxyapatite synthesized by hydrolysis using cetyltrimethylammonium bromide (CTAB) as a surfactant. J. Cryst. Growth 275, 2339–2344 (2005). https://doi.org/10.1016/j.jcrysgro.2004.11.330
A. Almirall, G. Larrecq, J.A. Delgado, S. Martínez, J.A. Planell, M.P. Ginebra, Fabrication of low temperature macroporous hydroxyapatite scaffolds by foaming and hydrolysis of an α-TCP paste. Biomaterials 25, 3671–3680 (2004). https://doi.org/10.1016/j.biomaterials.2003.10.066
M. Kavitha, R. Subramanian, K.S. Vinoth, R. Narayanan, G. Venkatesh, N. Esakkiraja, Optimization of process parameters for solution combustion synthesis of strontium substituted hydroxyapatite nanocrystals using design of experiments approach. Powder Technol. 271, 167–181 (2015). https://doi.org/10.1016/j.powtec.2014.10.046
J.S. Cho, J.C. Lee, S.H. Rhee, Effect of precursor concentration and spray pyrolysis temperature upon hydroxyapatite particle size and density. J. Biomed. Mater. Res. Part B Appl. Biomater. 104, 422–430 (2016). https://doi.org/10.1002/jbm.b.33406
S. Sasikumar, R. Vijayaraghavan, Synthesis and characterization of bioceramic calcium phosphates by rapid combustion synthesis. J. Mater. Sci. Technol. 26, 1114–1118 (2010). https://doi.org/10.1016/S1005-0302(11)60010-8
R. Ayers, N. Hannigan, N. Vollmer, C. Unuvar, Combustion synthesis of heterogeneous calcium phosphate bioceramics from calcium oxide and phosphate precursors. Int. J. Self Propag. High Temp. Synth. 20, 6–14 (2011). https://doi.org/10.3103/S1061386211010031
R. Ramakrishnan, P. Wilson, T. Sivakumar, I. Jemina, A comparative study of hydroxyapatites synthesized using various fuels through aqueous and alcohol mediated combustion routes. Ceram. Int. 39, 3519–3532 (2013). https://doi.org/10.1016/j.ceramint.2012.10.176
N. Wakiya, M. Yamasaki, T. Adachi, A. Inukai, N. Sakamoto, D. Fu, O. Sakurai, K. Shinozaki, H. Suzuki, Preparation of hydroxyapatite-ferrite composite particles by ultrasonic spray pyrolysis. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 173, 195–198 (2010). https://doi.org/10.1016/j.mseb.2009.12.013
W. Widiyastuti, A. Setiawan, S. Winardi, T. Nurtono, H. Setyawan, Particle formation of hydroxyapatite precursor containing two components in a spray pyrolysis process. Front. Chem. Sci. Eng. 8, 104–113 (2014). https://doi.org/10.1007/s11705-014-1406-1
G.H. An, H.J. Wang, B.H. Kim, Y.G. Jeong, Y.H. Choa, Fabrication and characterization of a hydroxyapatite nanopowder by ultrasonic spray pyrolysis with salt-assisted decomposition. Mater. Sci. Eng. A 449–451, 821–824 (2007). https://doi.org/10.1016/j.msea.2006.02.436
J.S. Cho, Y.C. Kang, Nano-sized hydroxyapatite powders prepared by flame spray pyrolysis. J. Alloys Compd. 464, 282–287 (2008). https://doi.org/10.1016/j.jallcom.2007.09.092
M.E. Zilm, L. Chen, V. Sharma, A. McDannald, M. Jain, R. Ramprasad, M. Wei, Hydroxyapatite substituted by transition metals: experiment and theory. Phys. Chem. Chem. Phys. 18, 16457–16465 (2016). https://doi.org/10.1039/c6cp00474a
M. Frasnelli, F. Cristofaro, V.M. Sglavo, S. Dirè, E. Callone, R. Ceccato, G. Bruni, A.I. Cornaglia, L. Visai, Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 71, 653–662 (2017). https://doi.org/10.1016/j.msec.2016.10.047
S.Y. Park, K.-I. Kim, S.P. Park, J.H. Lee, H.S. Jung, Aspartic acid-assisted synthesis of multifunctional strontium-substituted hydroxyapatite microspheres. Cryst. Growth Des. 16, 4318–4326 (2016). https://doi.org/10.1021/acs.cgd.6b00420
J. Li, L. Yang, X. Guo, W. Cui, S. Yang, J. Wang, Y. Qu, Z. Shao, S. Xu, Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomed. Mater. (2018). https://doi.org/10.1088/1748-605X/aa89af
J. Li, X. Liu, S. Park, A.L. Miller, A. Terzic, L. Lu, Strontium-substituted hydroxyapatite stimulates osteogenesis on poly(propylene fumarate) nanocomposite scaffolds. J. Biomed. Mater. Res. Part A 107, 631–642 (2019). https://doi.org/10.1002/jbm.a.36579
Z. Geng, X. Wang, J. Zhao, Z. Li, L. Ma, S. Zhu, Y. Liang, Z. Cui, H. He, X. Yang, The synergistic effect of strontium-substituted hydroxyapatite and microRNA-21 on improving bone remodeling and osseointegration. Biomater. Sci. 6, 2694–2703 (2018). https://doi.org/10.1039/c8bm00716k
F. Tamimi, N.D. Le, D.C. Bassett, S. Ibasco, U. Gbureck, J. Knowles, A. Wright, A. Flynn, S.V. Komarova, J.E. Barralet, Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomater. 7, 2678–2685 (2011). https://doi.org/10.1016/j.actbio.2011.02.007
A. Ewald, K. Helmschrott, G. Knebl, N. Mehrban, L.M. Grover, U. Gbureck, Effect of cold-setting calcium- and magnesium phosphate matrices on protein expression in osteoblastic cells. J Biomed Mater Res Part B Appl Biomater 96 B, 326–332 (2011). https://doi.org/10.1002/jbm.b.31771
N.C. Andrés, N.L. D’Elía, J.M. Ruso, A.E. Campelo, V.L. Massheimer, P.V. Messina, Manipulation of Mg2+-Ca2+ switch on the development of bone mimetic hydroxyapatite. ACS Appl. Mater. Interfaces 9, 15698–15710 (2017). https://doi.org/10.1021/acsami.7b02241
I. Uysal, F. Severcan, A. Tezcaner, Z. Evis, Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 24, 340–349 (2014). https://doi.org/10.1016/j.pnsc.2014.06.004
M. Irfan, S.N. Sultana, B. Venkateswarlu, M. Jagannatham, R. Dumpala, B.R. Sunil, Zinc-substituted hydroxyapatite: synthesis, structural analysis, and antimicrobial behavior. Trans. Indian Inst. Met. 74, 2335–2344 (2021). https://doi.org/10.1007/s12666-021-02290-x
E.S. Thian, J. Huang, S.M. Best, Z.H. Barber, W. Bonfield, Silicon-substituted hydroxyapatite: the next generation of bioactive coatings. Mater. Sci. Eng. C 27, 251–256 (2007). https://doi.org/10.1016/j.msec.2006.05.016
Y.O. Nikitina, N.V. Petrakova, A.A. Ashmarin, D.D. Titov, S.V. Shevtsov, T.N. Penkina, E.A. Kuvshinova, S.M. Barinov, V.S. Komlev, N.S. Sergeeva, Preparation and properties of copper-substituted hydroxyapatite powders and ceramics. Inorg. Mater. 55, 1061–1067 (2019). https://doi.org/10.1134/S002016851910011X
A. Elrayah, W. Zhi, S. Feng, S. Al-Ezzi, H. Lei, J. Weng, Preparation of micro/nano-structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration. Materials (Basel) (2018). https://doi.org/10.3390/ma11091516
J. Sang Cho, S.H. Um, D. Su Yoo, Y.C. Chung, S. Hye Chung, J.C. Lee, S.H. Rhee, Enhanced osteoconductivity of sodium-substituted hydroxyapatite by system instability. J. Biomed. Mater. Res. Part B Appl. Biomater. 102, 1046–1062 (2014). https://doi.org/10.1002/jbm.b.33087
W.E. Cabrera, I. Schrooten, M.E. De Broe, P.C. D’Haese, Strontium and bone. J. Bone Miner. Res. 14, 661–668 (1999). https://doi.org/10.1359/jbmr.1999.14.5.661
C. Li, O. Paris, S. Siegel, P. Roschger, E.P. Paschalis, K. Klaushofer, P. Fratzl, Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J. Bone Miner. Res. 25, 968–975 (2010). https://doi.org/10.1359/jbmr.091038
G. Boivin, D. Farlay, M.T. Khebbab, X. Jaurand, P.D. Delmas, P.J. Meunier, In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of mineralization. Osteoporos. Int. 21, 667–677 (2010). https://doi.org/10.1007/s00198-009-1005-z
P. Roschger, I. Manjubala, N. Zoeger, F. Meirer, R. Simon, C. Li, N. Fratzl-Zelman, B.M. Misof, E.P. Paschalis, C. Streli, P. Fratzl, K. Klaushofer, Bone material quality in transiliac bone biopsies of postmenopausal osteoporotic women after 3 years of strontium ranelate treatment. J. Bone Miner. Res. 25, 891–900 (2010). https://doi.org/10.1359/jbmr.091028
J. Christoffersen, M.R. Christoffersen, N. Kolthoff, O. Bärenholdt, Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone 20, 47–54 (1997). https://doi.org/10.1016/S8756-3282(96)00316-X
H. Zhu, D. Guo, L. Sun, H. Li, D.A.H. Hanaor, F. Schmidt, K. Xu, Nanostructural insights into the dissolution behavior of Sr-doped hydroxyapatite. J. Eur. Ceram. Soc. 38, 5554–5562 (2018). https://doi.org/10.1016/j.jeurceramsoc.2018.07.056
K. Zhu, K. Yanagisawa, R. Shimanouchi, A. Onda, K. Kajiyoshi, Preferential occupancy of metal ions in the hydroxyapatite solid solutions synthesized by hydrothermal method. J. Eur. Ceram. Soc. 26, 509–513 (2006). https://doi.org/10.1016/j.jeurceramsoc.2005.07.019
K. Kandori, M. Saito, H. Saito, A. Yasukawa, T. Ishikawa, Adsorption of protein on non-stoichiometric calcium-strontium hydroxyapatite. Colloids Surf. A Physicochem. Eng. Asp. 94, 225–230 (1995). https://doi.org/10.1016/0927-7757(94)02969-5
E. Landi, G. Logroscino, L. Proietti, A. Tampieri, M. Sandri, S. Sprio, Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 19, 239–247 (2008). https://doi.org/10.1007/s10856-006-0032-y
S. Gomes, G. Renaudin, E. Jallot, J.M. Nedelec, Structural characterization and biological fluid interaction of sol-gel-derived Mg-substituted biphasic calcium phosphate ceramics. ACS Appl. Mater. Interfaces 1, 505–513 (2009). https://doi.org/10.1021/am800162a
E. Landi, A. Tampieri, M. Mattioli-Belmonte, G. Celotti, M. Sandri, A. Gigante, P. Fava, G. Biagini, Biomimetic Mg- and Mg, CO3-substituted hydroxyapatites: synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 26, 2593–2601 (2006). https://doi.org/10.1016/j.jeurceramsoc.2005.06.040
S. Lala, M. Ghosh, P.K. Das, D. Das, T. Kar, S.K. Pradhan, Magnesium substitution in carbonated hydroxyapatite: structural and microstructural characterization by Rietveld’s refinement. Mater. Chem. Phys. 170, 319–329 (2016). https://doi.org/10.1016/j.matchemphys.2015.12.058
A.A. Chaudhry, J. Goodall, M. Vickers, J.K. Cockcroft, I. Rehman, J.C. Knowles, J.A. Darr, Synthesis and characterisation of magnesium substituted calcium phosphate bioceramic nanoparticles made via continuous hydrothermal flow synthesis. J. Mater. Chem. 18, 5900–5908 (2008). https://doi.org/10.1039/b807920j
A. Farzadi, F. Bakhshi, M. Solati-Hashjin, M. Asadi-Eydivand, N.A.A. Osman, Magnesium incorporated hydroxyapatite: synthesis and structural properties characterization. Ceram. Int. 40, 6021–6029 (2014). https://doi.org/10.1016/j.ceramint.2013.11.051
J.P. O’Connor, D. Kanjilal, M. Teitelbaum, S.S. Lin, J.A. Cottrell, Zinc as a therapeutic agent in bone regeneration. Materials (Basel) 13, 1–22 (2020). https://doi.org/10.3390/ma13102211
M. Yamaguchi, R. Yamaguchi, Action of zinc on bone metabolism in rats. Increases in alkaline phosphatase activity and DNA content. Biochem. Pharmacol. 35, 773–777 (1986). https://doi.org/10.1016/0006-2952(86)90245-5
C. Ergun, T.J. Webster, R. Bizios, R.H. Doremus, Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. I. Structure and microstructure. J. Biomed. Mater. Res. 59, 305–311 (2002). https://doi.org/10.1002/jbm.1246
F. Miyaji, Y. Kono, Y. Suyama, Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 40, 209–220 (2005). https://doi.org/10.1016/j.materresbull.2004.10.020
M. Li, X. Xiao, R. Liu, C. Chen, L. Huang, Structural characterization of zinc-substituted hydroxyapatite prepared by hydrothermal method. J. Mater. Sci. Mater. Med. 19, 797–803 (2008). https://doi.org/10.1007/s10856-007-3213-4
Z. Zhong, J. Qin, J. Ma, Rapid synthesis of citrate-zinc substituted hydroxyapatite using the ultrasonication-microwave method. Ceram. Int. 43, 13308–13313 (2017). https://doi.org/10.1016/j.ceramint.2017.07.029
E.S. Thian, T. Konishi, Y. Kawanobe, P.N. Lim, C. Choong, B. Ho, M. Aizawa, Zinc-substituted hydroxyapatite: a biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 24, 437–445 (2013). https://doi.org/10.1007/s10856-012-4817-x
E.M. Carlisle, Silicon: a requirement in bone formation independent of vitamin D1. Calcif. Tissue Int. 33, 27–34 (1981). https://doi.org/10.1007/BF02409409
W. Götz, E. Tobiasch, S. Witzleben, M. Schulze, Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 11, 1–27 (2019). https://doi.org/10.3390/pharmaceutics11030117
E.M. Carlisle, Silicon: a possible factor in bone calcification. Science 167(3916), 279–280 (1970). https://doi.org/10.1126/science.167.3916.279
L.T. Bang, B.D. Long, R. Othman, Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: synthesis, mechanical properties, and solubility evaluations. Sci. World J. (2014). https://doi.org/10.1155/2014/969876
A. Tsalsabila, Y.W. Sari, A. Maddu, Synthesis of silicon substituted hydroxyapatite using microwave irradiation, in: Proc—2018 1st Int Conf Bioinformatics, Biotechnol Biomed Eng BioMIC 2018, vol. 1, pp. 1–5 (2019). https://doi.org/10.1109/BIOMIC.2018.8610598
C. Palacios, The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 46, 621–628 (2006). https://doi.org/10.1080/10408390500466174
S. Shanmugam, B. Gopal, Copper substituted hydroxyapatite and fluorapatite: synthesis, characterization and antimicrobial properties. Ceram. Int. 40, 15655–15662 (2014). https://doi.org/10.1016/j.ceramint.2014.07.086
H.J. Kronzucker, D. Coskun, L.M. Schulze, J.R. Wong, D.T. Britto, Sodium as nutrient and toxicant. Plant Soil 369, 1–23 (2013). https://doi.org/10.1007/s11104-013-1801-2
R. Mangili, J.J. Bending, G. Scott, L.K. Li, A. Gupta, G. Viberti, Increased sodium-lithium countertransport activity in red cells of patients with insulin-dependent diabetes and nephropathy. N. Engl. J. Med. 318, 146–150 (1988)
K. Hu, B.R. Olsen, The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 91, 30–38 (2016). https://doi.org/10.1016/j.bone.2016.06.013
Funding
This research was supported by grants from the National Research Foundation of Korea funded by the Ministry of Science and ICT for Bio-inspired Innovation Technology Development Project (NRF-2018M3C1B7021997) and Basic Science Research Program (NRF-2020R1A2C2006100). Dr. Jun Hyuk Heo and Mr. Si Hyun Kim appreciate the support of the National Research Foundation of Korea funded by the Ministry of Education (NRF-2022R1C1C2002823 (JHH) and NRF-2020R1A6A3A13070482 (SHK)).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S.H., Park, C.H., Heo, J.H. et al. Progress and perspectives of metal-ion-substituted hydroxyapatite for bone tissue engineering: comparison with hydroxyapatite. J. Korean Ceram. Soc. 59, 271–288 (2022). https://doi.org/10.1007/s43207-022-00198-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00198-w