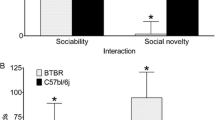
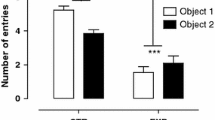
Abstract
Febrile seizure (FS) is one of the most prevalent etiological events in childhood affecting 2–5% of children from 3 months to 5 years old. Debates on whether neurodevelopmental consequences rise in later life following a febrile seizure or not are still ongoing however there is limited evidence of its effect, especially in a laboratory setting. Moreover, the comparative study using both male and female animal models is sparse. To examine the effect of FS on the behavioral features of mice, both sexes of ICR mice were induced with hyperthermic seizures through exposure to an infrared heat lamp. The mice were divided into two groups, one receiving a single febrile seizure at postnatal day 11 (P11) and one receiving three FS at P11, P13, and P15. Starting at P30 the FS-induced mice were subjected to a series of behavioral tests. Mice with seizures showed no locomotor and motor coordination deficits, repetitive, and depressive-like behavior. However, the FS-induced mice showed impulsive-like behavior in both elevated plus maze and cliff avoidance tests, which is more prominent in male mice. A greater number of mice displayed impaired CAT in both males and females in the three-time FS-induced group compared to the single induction group. These results demonstrate that after induction of FS, male mice have a higher susceptibility to consequences of febrile seizure than female mice and recurrent febrile seizure has a higher chance of subsequent disorders associated with decreased anxiety and increased impulsivity. We confirmed the dysregulated expression of impulsivity-related genes such as 5-HT1A and tryptophan hydroxylase 2 from the prefrontal cortices of FS-induced mice implying that the 5-HT system would be one of the mechanisms underlying the increased impulsivity after FS. Taken together, these findings are useful in unveiling future discoveries about the effect of childhood febrile seizure and the mechanism behind it.










Similar content being viewed by others
References
Major P, Thiele EA (2007) Seizures in children: determining the variation. Pediatr Rev 28:363–371. https://doi.org/10.1542/pir.28-10-363
Shinnar S et al (2012) MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology 79:871–877. https://doi.org/10.1212/WNL.0b013e318266fcc5
Lewis DV et al (2014) Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann Neurol 75:178–185. https://doi.org/10.1002/ana.24081
Sharawat IK et al (2016) Evaluation of risk factors associated with first episode febrile seizure. J Clin Diagn Res 10:SC10–SC13. https://doi.org/10.7860/JCDR/2016/18635.7853
Christensen KJ et al (2021) Birth characteristics and risk of febrile seizures. Acta Neurol Scand 144:51–57. https://doi.org/10.1111/ane.13420
Canpolat M et al (2018) Investigating the prevalence of febrile convulsion in Kayseri, Turkey: an assessment of the risk factors for recurrence of febrile convulsion and for development of epilepsy. Seizure 55:36–47. https://doi.org/10.1016/j.seizure.2018.01.007
Seinfeld DS, Pellock JM (2013) Recent research on febrile seizures: a review. J Neurol Neurophysiol. https://doi.org/10.4172/2155-9562.1000165
Ellenberg JH, Nelson KB (1978) Febrile seizures and later intellectual performance. Arch Neurol 35:17–21. https://doi.org/10.1001/archneur.1978.00500250021004
Verity CM, Greenwood R, Golding J (1998) Long-term intellectual and behavioral outcomes of children with febrile convulsions. N Engl J Med 338:1723–1728. https://doi.org/10.1056/NEJM199806113382403
Arico M et al (2020) ADHD and ADHD-related neural networks in benign epilepsy with centrotemporal spikes: a systematic review. Epilepsy Behav 112:107448. https://doi.org/10.1016/j.yebeh.2020.107448
Bertelsen EN et al (2016) Childhood epilepsy, febrile seizures, and subsequent risk of ADHD. Pediatrics 138:e20154654. https://doi.org/10.1542/peds.2015-4654
Kim EH et al (2014) Attention-deficit/hyperactivity disorder and attention impairment in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 37:54–58. https://doi.org/10.1016/j.yebeh.2014.05.030
Ku YC et al (2014) Risk of subsequent attention deficit-hyperactivity disorder in children with febrile seizures. Arch Dis Child 99:322–326. https://doi.org/10.1136/archdischild-2013-304647
Tu YF et al (2014) Febrile convulsions increase risk of Tourette syndrome. Seizure 23:651–656. https://doi.org/10.1016/j.seizure.2014.05.005
Alese OO et al (2020) Prolonged febrile seizure history exacerbates seizure severity in a pentylenetetrazole rat model of epilepsy. Brain Res Bull 155:137–144. https://doi.org/10.1016/j.brainresbull.2019.11.021
Patterson KP et al (2015) Rapid, coordinate inflammatory responses after experimental febrile status epilepticus: implications for epileptogenesis. eNeuro. https://doi.org/10.1523/ENEURO.0034-15.2015
Crespo M, Leon-Navarro DA, Martin M (2021) Glutamatergic system is affected in brain from an hyperthermia-induced seizures rat model. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-021-01041-2
Kasahara Y, Ikegaya Y, Koyama R (2018) Neonatal seizure models to study epileptogenesis. Front Pharmacol 9:385. https://doi.org/10.3389/fphar.2018.00385
Sanon NT, Desgent S, Carmant L (2012) Atypical febrile seizures, mesial temporal lobe epilepsy, and dual pathology. Epilepsy Res Treat 2012:342928. https://doi.org/10.1155/2012/342928
Dube CM et al (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30:7484–7494. https://doi.org/10.1523/Jneurosci.0551-10.2010
Eun BL et al (2015) Lipopolysaccharide potentiates hyperthermia-induced seizures. Brain Behav 5:e00348. https://doi.org/10.1002/brb3.348
Lemmens EM et al (2005) Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia 46:1603–1612. https://doi.org/10.1111/j.1528-1167.2005.00252.x
Lugo JN, Swann JW, Anderson AE (2014) Early-life seizures result in deficits in social behavior and learning. Exp Neurol 256:74–80. https://doi.org/10.1016/j.expneurol.2014.03.014
Auvin S et al (2009) Inflammation in rat pups subjected to short hyperthermic seizures enhances brain long-term excitability. Epilepsy Res 86:124–130. https://doi.org/10.1016/j.eplepsyres.2009.05.010
Westmark CJ et al (2008) Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. Int J Clin Exp Pathol 1:157–168. PMID: 18784809
Kraeuter A-K, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice, in pre-clinical models. Springer, Berlin, pp 105–111. https://doi.org/10.1007/978-1-4939-8994-2_10
Wolf A et al (2016) A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS ONE 11:e0147733. https://doi.org/10.1371/journal.pone.0147733
Bak J et al (2017) Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation. Behav Brain Res 320:219–224. https://doi.org/10.1016/j.bbr.2016.12.011
Yankelevitch-Yahav R et al (2015) The forced swim test as a model of depressive-like behavior. Jove-J Vis Exp 97:e52587. https://doi.org/10.3791/52587
Shiotsuki H et al (2010) A rotarod test for evaluation of motor skill learning. J Neurosci Methods 189:180–185. https://doi.org/10.1016/j.jneumeth.2010.03.026
Yamashita M et al (2013) Impaired cliff avoidance reaction in dopamine transporter knockout mice. Psychopharmacology 227:741–749. https://doi.org/10.1007/s00213-013-3009-9
Chen X et al (2015) Impairment of oligodendroglia maturation leads to aberrantly increased cortical glutamate and anxiety-like behaviors in juvenile mice. Front Cell Neurosci 9:467. https://doi.org/10.3389/fncel.2015.00467
Kim S, Lee D (2011) Prefrontal cortex and impulsive decision making. Biol Psychiatry 69:1140–1146. https://doi.org/10.1016/j.biopsych.2010.07.005
Semple BD et al (2013) Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. https://doi.org/10.1016/j.pneurobio.2013.04.001
Dutta S, Sengupta P (2016) Men and mice: relating their ages. Life Sci 152:244–248. https://doi.org/10.1016/j.lfs.2015.10.025
Brenhouse HC, Schwarz JM (2016) Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev 70:288–299. https://doi.org/10.1016/j.neubiorev.2016.05.035
Patros CH et al (2016) Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev 43:162–174. https://doi.org/10.1016/j.cpr.2015.11.001
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361. https://doi.org/10.1007/pl00005481
Dalley JW et al (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260. https://doi.org/10.1016/j.pbb.2007.12.021
Matsuoka Y et al (2005) Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci USA 102:16066–16071. https://doi.org/10.1073/pnas.0504908102
Gershon J (2002) A meta-analytic review of gender differences in ADHD. J Atten Disord 5:143–154. https://doi.org/10.1177/108705470200500302
Castelhano AS et al (2010) Social play impairment following status epilepticus during early development. J Neural Transm (Vienna) 117:1155–1160. https://doi.org/10.1007/s00702-010-0460-1
Desgent S et al (2012) Early-life stress is associated with gender-based vulnerability to epileptogenesis in rat pups. PLoS ONE. https://doi.org/10.1371/journal.pone.0042622
Akman O, Moshe SL, Galanopoulou AS (2014) Sex-specific consequences of early life seizures. Neurobiol Dis 72:153–166. https://doi.org/10.1016/j.nbd.2014.05.021
Bernard PB et al (2013) Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis 59:1–17. https://doi.org/10.1016/j.nbd.2013.06.013
Cornejo BJ et al (2007) A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol 61:411–426. https://doi.org/10.1002/ana.21071
Karnam HB et al (2009) Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol 217:378–387. https://doi.org/10.1016/j.expneurol.2009.03.028
Fliers EA et al (2010) Undertreatment of motor problems in children with ADHD. Child Adolesc Mental Health 15:85–90. https://doi.org/10.1111/j.1475-3588.2009.00538.x
Bevilacqua L, Goldman D (2013) Genetics of impulsive behaviour. Philos Trans R Soc Lond B Biol Sci 368:20120380. https://doi.org/10.1098/rstb.2012.0380
Schreiber R, De Vry J (1993) 5-HT1A receptor ligands in animal models of anxiety, impulsivity and depression: multiple mechanisms of action? Prog Neuropsychopharmacol Biol Psychiatry 17:87–104. https://doi.org/10.1016/0278-5846(93)90034-p
Chiavegatto S et al (2001) Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 98:1277–1281. https://doi.org/10.1073/pnas.98.3.1277
Angoa-Pérez M et al (2012) Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem 121:974–984. https://doi.org/10.1111/j.1471-4159.2012.07739.x
Jupp B et al (2013) Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur J Neurosci 37:1519–1528. https://doi.org/10.1111/ejn.12146
Harrison AA, Everitt BJ, Robbins TW (1997) Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology 133:329–342. https://doi.org/10.1007/s002130050410
Robbins T, Dalley J (2017) Impulsivity, risky choice, and impulse control disorders: animal models. Decision neuroscience. Elsevier, Oxford, pp 81–93. https://doi.org/10.1016/B978-0-12-805308-9.00007-5
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395. https://doi.org/10.1016/j.cpr.2006.01.001
Toczek MT et al (2003) PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology 60:749–756. https://doi.org/10.1212/01.wnl.0000049930.93113.20
Merlet I et al (2004) 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain 127:900–913. https://doi.org/10.1093/brain/awh109
Richerson GB, Buchanan GF (2011) The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia 52(Suppl 1):28–38. https://doi.org/10.1111/j.1528-1167.2010.02908.x
Assem-Hilger E et al (2010) Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-11C] WAY-100635 PET study. Epilepsy Behav 19:467–473. https://doi.org/10.1016/j.yebeh.2010.07.030
Martinez A et al (2013) The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology 80:1465–1471. https://doi.org/10.1212/WNL.0b013e31828cf809
Economidou D et al (2012) Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology 37:2057–2066. https://doi.org/10.1038/npp.2012.53
Krause J (2008) SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Rev Neurother 8:611–625. https://doi.org/10.1586/14737175.8.4.611
Brown AB et al (2010) Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 153B:365–375. https://doi.org/10.1002/ajmg.b.31022
van der Kooij MA, Glennon JC (2007) Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev 31:597–618. https://doi.org/10.1016/j.neubiorev.2006.12.002
Schubert J et al (2014) Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat Genet 46:1327–1332. https://doi.org/10.1038/ng.3130
Feenstra B et al (2014) Common variants associated with general and MMR vaccine-related febrile seizures. Nat Genet 46:1274–1282. https://doi.org/10.1038/ng.3129
Skotte L et al (2022) Genome-wide association study of febrile seizures implicates fever response and neuronal excitability genes. Brain. https://doi.org/10.1093/brain/awab260
Kasahara Y et al (2019) The pharmacological assessment of GABAA receptor activation in experimental febrile seizures in mice. eNeuro. https://doi.org/10.1523/ENEURO.0429-18.2019
Rakgantsho C, Mabandla MV (2019) Acetylcholine receptor agonist effect on seizure activity and GABAergic mechanisms involved in prolonged febrile seizure development in an animal model. Brain Res Bull 149:203–207. https://doi.org/10.1016/j.brainresbull.2019.04.022
Hayes DJ et al (2014) Brain γ-aminobutyric acid: a neglected role in impulsivity. Eur J Neurosci 39:1921–1932. https://doi.org/10.1111/ejn.12485
Murphy ER et al (2012) Impulsive behaviour induced by both NMDA receptor antagonism and GABA(A) receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology 219:401–410. https://doi.org/10.1007/s00213-011-2572-1
Keele NB (2005) The role of serotonin in impulsive and aggressive behaviors associated with epilepsy-like neuronal hyperexcitability in the amygdala. Epilepsy Behav 7:325–335. https://doi.org/10.1016/j.yebeh.2005.06.014
Pape HC et al (2010) Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology 58:29–34. https://doi.org/10.1016/j.neuropharm.2009.06.001
Reinscheid RK, Xu YL (2005) Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J 272:5689–5693. https://doi.org/10.1111/j.1742-4658.2005.04982.x
Acknowledgements
This paper was supported by Konkuk University in 2020.
Funding
This paper was supported by Konkuk University in 2020.
Author information
Authors and Affiliations
Contributions
CGR, ELG, and KJA performed the behavioral experiments. SJJ verified the analytical methods. CYS conceived of the presented idea and also supervised the findings of this work. CGR, SJJ, and CYS wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Remonde, C.G., Gonzales, E.L., Adil, K.J. et al. Augmented impulsive behavior in febrile seizure-induced mice. Toxicol Res. 39, 37–51 (2023). https://doi.org/10.1007/s43188-022-00145-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-022-00145-1