Abstract
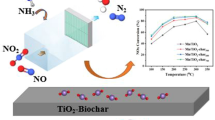
Gasification technology can effectively realize energy recovery from municipal solid waste (MSW) to reduce its negative impact on the environment. However, ammonia, as a pollutant derived from MSW gasification, needs to be treated because its emission is considered harmful to mankind. This work aims to decompose the NH3 pollutant from MSW gasification by an in-situ catalytic method. The MSW sample is composed of rice, paper, polystyrene granules, rubber gloves, textile and wood chips. Ni–M (M=Co, Fe, Zn) bimetallic catalysts supported on sewage sludge-derived biochar (SSC) were prepared by co-impregnation method and further characterized by X-ray diffraction, N2 isothermal adsorption, scanning electron microscopy, transmission electron microscopy and NH3 temperature programmed desorption. Prior to the experiments, the catalysts were first homogeneously mixed with the MSW sample, and then in-situ catalytic tests were conducted in a horizontal fixed-bed reactor. The effect of the second metal (Co, Fe, Zn) on the catalytic performance was compared to screen the best Ni-M dual. It was found that the Ni–Co/SSC catalyst had the best activity toward NH3 decomposition, whose decomposition rate reached 40.21% at 650 °C. The best catalytic performance of Ni–Co/SSC can be explained by its smaller Ni particle size that facilitates the dispersion of active sites as well as the addition of Co reducing the energy barrier for the associative decomposition of NH species during the NH3 decomposition process. Besides, the activity of Ni–Co/SSC increased from 450 °C to 700 °C as the NH3 decomposition reaction was endothermic.









Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Yu, J., Sun, L., Wang, B., et al. 2016. Study on the behavior of heavy metals during thermal treatment of municipal solid waste (MSW) components. Environmental science and pollution research international 23: 253–265.
Cerda, A., Artola, A., Font, X., et al. 2018. Composting of food wastes: status and challenges. Bioresource Technology 248: 57–67.
Shah, A.V., Srivastava, V.K., Mohanty, S.S., et al. 2021. Municipal solid waste as a sustainable resource for energy production: State-of-the-art review. Journal of Environmental Chemical Engineering 9: 105717.
Nanda, S., and Berruti, F. 2021. A technical review of bioenergy and resource recovery from municipal solid waste. Journal of Hazardous Materials 403: 123970.
Wei, J., Li, H., Liu, J., et al. 2022. National and provincial dioxin emissions from municipal solid waste incineration in China. Science of the Total Environment 851: 158128.
Chuai, X., Yang, Q., Zhang, T., et al. 2022. Speciation and leaching characteristics of heavy metals from municipal solid waste incineration fly ash. Fuel 328: 125338.
Makarichi, L., Jutidamrongphan, W., and Techato, K.A. 2018. The evolution of waste-to-energy incineration: A review. Renewable and Sustainable Energy Reviews 91: 812–821.
Yang, Y., Liew, R.K., Tamothran, A.M., et al. 2021. Gasification of refuse-derived fuel from municipal solid waste for energy production: A review. Environmental Chemistry Letters 19: 2127–2140.
Arena, U. 2012. Process and technological aspects of municipal solid waste gasification review. Waste Management 32: 625–639.
Lee, D.J. 2022. Gasification of municipal solid waste (MSW) as a cleaner final disposal route: a mini-review. Bioresour Technology 344: 126217.
Juutilainen, S.J., Simell, P.A., and Krause, A.O.I. 2006. Zirconia: Selective oxidation catalyst for removal of tar and ammonia from biomass gasification gas. Applied Catalysis B Environmental 62: 86–92.
Xu, C., Donald, J., Byambajav, E., et al. 2010. Recent advances in catalysts for hot-gas removal of tar and NH3 from biomass gasification. Fuel 89: 1784–1795.
Wilk, V., Kitzler, H., Koppatz, S., et al. 2011. Gasification of waste wood and bark in a dual fluidized bed steam gasifier. Biomass Conversion and Biorefinery 1: 91–97.
Jiang, Q., Li, T., He, Y., et al. 2022. Simultaneous removal of hydrogen sulfide and ammonia in the gas phase: A review. Environmental Chemistry Letters 20: 1403–1419.
Gu, Y., Jin, Z., Zhang, H., et al. 2015. Transition metal nanoparticles dispersed in an alumina matrix as active and stable catalysts for COx-free hydrogen production from ammonia. Journal of Materials Chemistry 3: 17172–17180.
Lamb, K.E., Dolan, M.D., and Kennedy, D.F. 2019. Ammonia for hydrogen storage: A review of catalytic ammonia decomposition and hydrogen separation and purification. International Journal of Hydrogen Energy 44: 3580–3593.
Le, T.A., Kim, Y., Kim, H.W., et al. 2021. Ru-supported lanthania-ceria composite as an efficient catalyst for COx-free H2 production from ammonia decomposition. Applied Catalysis B: Environmental 285: 119831.
Lucentini, I., Colli, G.G., Luzi, C.D., et al. 2021. Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: Effect of metal loading and kinetic analysis. Applied Catalysis B: Environmental 286: 119896.
Pinzon, M., Romero, A., Consuegra, A.D., et al. 2021. Hydrogen production by ammonia decomposition over ruthenium supported on SiC catalyst. Journal of Industrial and Engineering Chemistry 94: 326–335.
Ji, J., Duan, X., Qian, G., et al. 2013. Fe particles on the tops of carbon nanofibers immobilized on structured carbon microfibers for ammonia decomposition. Catalysis Today 216: 254–260.
Choi, Y.K., Cho, M.H., and Kim, J.S. 2016. Air gasification of dried sewage sludge in a two-stage gasifier. Part 4: application of additives including Ni-impregnated activated carbon for the production of a tar-free and H2-rich producer gas with a low NH3 content. International Journal of Hydrogen Energy 41: 1460–1467.
Simonsen, S.B., Chakraborty, D., Chorkendorff, I., et al. 2012. Alloyed Ni-Fe nanoparticles as catalysts for NH3 decomposition. Applied Catalysis A: General 447: 22–31.
Yin, S., Zhang, Q., Xu, B., et al. 2004. Investigation on the catalysis of COx-free hydrogen generation from ammonia. Journal of Catalysis. 224: 384–396.
Lu, A., Nitz, J.J., Comotti, M., et al. 2010. Spatially and size selective synthesis of Fe-based nanoparticles on ordered mesoporous supports as highly active and stable catalysts for ammonia decomposition. Journal of the American Chemical Society 132: 14152–14162.
Varisli, D., Korkusuz, C., and Dogu, T. 2017. Microwave-assisted ammonia decomposition reaction over iron incorporated mesoporous carbon catalysts. Applied Catalysis B: Environmental 201: 370–380.
Ren, S., Huang, F., Zheng, J., et al. 2017. Ruthenium supported on nitrogen-doped ordered mesoporous carbon as highly active catalyst for NH3 decomposition to H2. International Journal of Hydrogen Energy 42: 5105–5113.
Zeng, Q., Tang, Nvzhi, Chen, H., et al. 2020. Application research of municipal sludge treatment and disposal technology. IOP Conference Series: Earth and Environmental Science 567: 012023.
Dai, L., Zhao, W., Zhang, H., et al. 2020. Research progress on adsorption of heavy metals by sewage sludge-based biochar in water. Environment Engineering 38: 70–77 ((in Chinese)).
Jung, S.Y., Lee, S.J., Park, J.J., et al. 2008. The simultaneous removal of hydrogen sulfide and ammonia over zinc-based dry sorbent supported on alumina. Separation and Purification Technology 63: 297–302.
Li, G., Zhang, H., Yu, X., et al. 2022. Highly efficient Co/NC catalyst derived from ZIF-67 for hydrogen generation through ammonia decomposition. International Journal of Hydrogen Energy 47: 12882–12892.
Boisen, A., Dahl, S., Norskov, J., et al. 2005. Why the optimal ammonia synthesis catalyst is not the optimal ammonia decomposition catalyst. Journal of Catalysis 230: 309–312.
Wu, Z., Li, X., Qin, Y., et al. 2020. Ammonia decomposition over SiO2-supported Ni-Co bimetallic catalyst for COx-free hydrogen generation. International Journal of Hydrogen Energy 45: 15263–15269.
Wang, F., Zeng, X., Wang, Y., et al. 2015. Investigation on in/ex-situ coal char gasification kinetics in a micro fluidized bed reactor. Journal of Fuel Chemistry and Technology 43: 393–401 ((in Chinese)).
Hashemnejad, S.M., and Parvari, M. 2011. Deactivation and regeneration of nickel-based catalysts for steam-methane reforming. Chinese Journal of Catalysis 32: 273–279.
Wu, H., Cao, A. 2013. Preparation and adding methods of Nessler's Reagent having effects on determination of water quality ammonia nitrogen. Advanced Materials Research 726-731: 1362–1366
Khan, W.U., Alasiri, H.S., Ali, S.A. et al. 2022. Recent advances in bimetallic catalysts for hydrogen production from ammonia. Chemical Record 22 (7): e202200030.
Fu, E., Qiu, Y., Lu, H., et al. 2021. Enhanced NH3 decomposition for H2 production over bimetallic M(M=Co, Fe, Cu)Ni/Al2O3. Fuel Processing Technology 221: 106945.
Dong, X., Jin, B., Cao, S., et al. 2020. COx co-methanation over coal combustion fly ash supported Ni-Re bimetallic catalyst: transformation from hazardous to high value-added products. Journal of Hazardous Materials 396: 122668.
Hu, X., Wang, W., Jin, Z., et al. 2019. Transition metal nanoparticles supported La-promoted MgO as catalysts for hydrogen production via catalytic decomposition of ammonia. Journal of Energy Chemistry 38: 41–49.
Qiu, J., Peng, Y., Tang, M., et al. 2022. Solvothermal preparation of Mn-based catalysts for simultaneous removal of 1,2-dichlorobenzene and furan. Waste Disposal & Sustainable Energy 4: 105–116.
Takahashi, A., and Fujitani, T. 2016. Kinetic analysis of decomposition of ammonia over nickel and ruthenium catalysts. Journal of Chemical Engineering of Japan 49: 22–28.
Yeo, S.C., Han, S.S., and Lee, H.M. 2014. Mechanistic investigation of the catalytic decomposition of ammonia (NH3) on an Fe (100) Surface: A DFT Study. Journal of Physical Chemistry C 118: 5309–5316.
Yi, K., Liu, H., Wang, J., et al. 2019. The adsorption and transformation of SO2, H2S and NH3 by using sludge gasification ash: effects of Fenton oxidation and CaO pre-conditioning. Chemical Engineering Journal 360: 1498–1508.
Mauerhofer, A.M., Fuchs, J., Muller, S., et al. 2019. CO2 gasification in a dual fluidized bed reactor system: impact on the product gas composition. Fuel 253: 1605–1616.
Qiu, S., Ren, T., and Li, J. 2018. The latest advances in the modified catalysts for hydrogen production from ammonia decomposition. Chemical Industry and Engineering Progress 37: 1001–1007 ((in Chinese)).
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2019YFC1906803) and Key R&D Program of Jiangsu Province (No. BE2021701).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, X., Huang, Y., Dong, X. et al. In-situ catalytic decomposition of emitted ammonia from municipal solid waste gasification by Ni–M bimetallic catalysts supported on sewage sludge-derived biochar. Waste Dispos. Sustain. Energy 5, 113–124 (2023). https://doi.org/10.1007/s42768-022-00124-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-022-00124-0