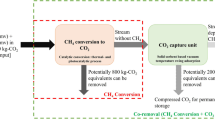
Abstract
Considering the global objective to mitigate climate change, import efforts are made on decreasing the net emission of CO2 from gas effluents. On the one hand CO2 capture—for example by adsorption onto solid basic materials—allows to withdraw CO2 from the waste gas streams emitted by incinerators, cement manufacture plants, combustion plants, power plants, etc. On the second hand, CO2 can be converted to useful chemicals—e.g. hydrogenation to methane—using appropriate heterogeneous catalysts. A relatively innovative strategy consists in combining both technologies by designing materials and processes which can switch between capture and methanation modes cyclically. This allows treating complex waste gas effluents by selectively and reversibly capturing CO2, and to perform the catalytic hydrogenation in appropriate reaction conditions. This short review presents the main strategies recently reported in the literature for such combined CO2 capture and methanation (CCCM) processes. We discuss the different types of reactor configurations and we present the formulations used in this context as adsorbent, as methanation catalysts, and as “dual functional materials”.


Reproduced from Veselovskaya et al. [63] with authorization from Springer



Similar content being viewed by others
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
References
Friedlingstein P, Andrew RM, Rogelj J, Peters GP, Canadell JG, Knutti R, et al. Persistent growth of CO2 emissions and implications for reaching climate targets. Nat Geosci. 2014;7:709.
Rogelj J, den Elzen M, Höhne N, Fransen T, Fekete H, Winkler H, et al. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature. 2016;534:631.
Koytsoumpa EI, Bergins C, Kakaras E. The CO2 economy: review of CO2 capture and reuse technologies. J Supercrit Fluids. 2018;132:3–16.
Rahman FA, Aziz MMA, Saidur R, Bakar WAWA, Hainin MR, Putrajaya R, et al. Pollution to solution: capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew Sustain Energy Rev. 2017;71:112–26.
Rafiee A, Rajab Khalilpour K, Milani D, Panahi M. Trends in CO2 conversion and utilization: a review from process systems perspective. J Environ Chem Eng. 2018;6(5):5771–94.
Al-Mamoori A, Krishnamurthy A, Rownaghi AA, Rezaei F. Carbon capture and utilization update. Energy Technol. 2017;5(6):834–49.
Song C, Liu Q, Ji N, Deng S, Zhao J, Li Y, et al. Alternative pathways for efficient CO2 capture by hybrid processes—a review. Renew Sustain Energy Rev. 2018;82:215–31.
Yu C-H, Huang C-H, Tan C-S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual Res. 2012;12(5):745–69.
Wang Q, Luo J, Zhong Z, Borgna A. CO2 capture by solid adsorbents and their applications: current status and new trends. Energy Environ Sci. 2011;4(1):42–55.
Alonso A, Moral-Vico J, Abo Markeb A, Busquets-Fité M, Komilis D, Puntes V, et al. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci Total Environ. 2017;595:51–62.
Sun H, Wu C, Shen B, Zhang X, Zhang Y, Huang J. Progress in the development and application of CaO-based adsorbents for CO2 capture—a review. Mater Today Sustain. 2018;1–2:1–27.
Estevez L, Barpaga D, Zheng J, Sabale S, Patel RL, Zhang J-G, et al. Hierarchically porous carbon materials for CO2 capture: the role of pore structure. Ind Eng Chem Res. 2018;57(4):1262–8.
Zhao Q, Wu F, Xie K, Singh R, Zhao J, Xiao P, et al. Synthesis of a novel hybrid adsorbent which combines activated carbon and zeolite NaUSY for CO2 capture by electric swing adsorption (ESA). Chem Eng J. 2018;336:659–68.
Pham T-H, Lee B-K, Kim J, Lee C-H. Enhancement of CO2 capture by using synthesized nano-zeolite. J Taiwan Inst Chem Eng. 2016;64:220–6.
Debecker DP, Gaigneaux EM, Busca G. Exploring, tuning, and exploiting the basicity of hydrotalcites for applications in heterogeneous catalysis. Chem Eur J. 2009;15(16):3920–35.
Darunte LA, Walton KS, Sholl DS, Jones CW. CO2 capture via adsorption in amine-functionalized sorbents. Curr Opin Chem Eng. 2016;12:82–90.
Liang Y, Harrison DP, Gupta RP, Green DA, McMichael WJ. Carbon dioxide capture using dry sodium-based sorbents. Energy Fuels. 2004;18(2):569–75.
Hicks JC, Drese JH, Fauth DJ, Gray ML, Qi G, Jones CW. Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly. J Am Chem Soc. 2008;130(10):2902–3.
Yu J, Xie L-H, Li J-R, Ma Y, Seminario JM, Balbuena PB. CO2 capture and separations Using MOFs: computational and experimental studies. Chem Rev. 2017;117(14):9674–754.
An J, Rosi NL. Tuning MOF CO2 adsorption properties via cation exchange. J Am Chem Soc. 2010;132(16):5578–9.
Liu J, Thallapally PK, McGrail BP, Brown DR, Liu J. Progress in adsorption-based CO2 capture by metal–organic frameworks. Chem Soc Rev. 2012;41(6):2308–22.
Trickett CA, Helal A, Al-Maythalony BA, Yamani ZH, Cordova KE, Yaghi OM. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat Rev Mater. 2017;2:17045.
Patil RS, Banerjee D, Zhang C, Thallapally PK, Atwood JL. Selective CO2 adsorption in a supramolecular organic framework. Angew Chem. 2016;128(14):4599–602.
Wang G, Leus K, Zhao S, Van Der Voort P. Newly designed covalent triazine framework based on novel n-heteroaromatic building blocks for efficient CO2 and H2 capture and storage. ACS Appl Mater Interfaces. 2018;10(1):1244–9.
Forse AC, Milner PJ, Lee J-H, Redfearn HN, Oktawiec J, Siegelman RL, et al. Elucidating CO2 chemisorption in diamine-appended metal-organic frameworks. J Am Chem Soc. 2018;140(51):18016–31.
Ram Reddy MK, Xu ZP, Lu GQ, da Costa DJC. Layered double hydroxides for CO2 Capture: structure evolution and regeneration. Ind Eng Chem Res. 2006;45(22):7504–9.
Artz J, Müller TE, Thenert K, Kleinekorte J, Meys R, Sternberg A, et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem Rev. 2018;118(2):434–504.
Pan S-Y, Chiang P-C, Pan W, Kim H. Advances in state-of-art valorization technologies for captured CO2 toward sustainable carbon cycle. Crit Rev Environ Sci Technol. 2018;48(5):471–534.
Centi G, Quadrelli EA, Perathoner S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ Sci. 2013;6(6):1711–31.
Kim A, Debecker DP, Devred F, Dubois V, Sanchez C, Sassoye C. CO2 methanation on Ru/TiO2 catalysts: on the effect of mixing anatase and rutile TiO2 supports. Appl Catal B. 2018;220:615–25.
Stangeland K, Kalai D, Li H, Yu Z. CO2 methanation: the effect of catalysts and reaction conditions. Energy Proc. 2017;105:2022–7.
Martins J, Batail N, Silva S, Rafik-Clement S, Karelovic A, Debecker DP, et al. CO2 hydrogenation with shape-controlled Pd nanoparticles embedded in mesoporous silica: elucidating stability and selectivity issues. Catal Commun. 2015;58:11–5.
Picasso CV, Safin DA, Dovgaliuk I, Devred F, Debecker DP, Li H-W, et al. Reduction of CO2 with KBH4 in solvent-free conditions. Int J Hydrog Energy. 2016;41(32):14377–86.
Rönsch S, Schneider J, Matthischke S, Schlüter M, Götz M, Lefebvre J, et al. Review on methanation—from fundamentals to current projects. Fuel. 2016;166:276–96.
Aziz MAA, Jalil AA, Triwahyono S, Ahmad A. CO2 methanation over heterogeneous catalysts: recent progress and future prospects. Green Chem. 2015;17(5):2647–63.
Vogt C, Monai M, Kramer GJ, Weckhuysen BM. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat Catal. 2019;2(3):188–97.
Younas M, Loong Kong L, Bashir MJK, Nadeem H, Shehzad A, Sethupathi S. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels. 2016;30(11):8815–31.
Goeppert A, Czaun M, Jones J-P, Surya Prakash GK, Olah GA. Recycling of carbon dioxide to methanol and derived products—closing the loop. Chem Soc Rev. 2014;43(23):7995–8048.
Karelovic A, Galdames G, Medina JC, Yévenes C, Barra Y, Jiménez R. Mechanism and structure sensitivity of methanol synthesis from CO2 over SiO2-supported Cu nanoparticles. J Catal. 2019;369:415–26.
Studt F, Sharafutdinov I, Abild-Pedersen F, Elkjær CF, Hummelshøj JS, Dahl S, et al. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat Chem. 2014;6(4):320–4.
Albrecht M, Rodemerck U, Schneider M, Bröring M, Baabe D, Kondratenko EV. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl Catal B. 2017;204:119–26.
Visconti CG, Martinelli M, Falbo L, Infantes-Molina A, Lietti L, Forzatti P, et al. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst. Appl Catal B. 2017;200:530–42.
Gao P, Li S, Bu X, Dang S, Liu Z, Wang H, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat Chem. 2017;9:1019.
Li W, Wang H, Jiang X, Zhu J, Liu Z, Guo X, et al. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018;8(14):7651–69.
Marques Mota F, Kim DH. From CO2 methanation to ambitious long-chain hydrocarbons: alternative fuels paving the path to sustainability. Chem Soc Rev. 2019;48:205–59.
Saeidi S, Najari S, Fazlollahi F, Nikoo MK, Sefidkon F, Klemeš JJ, et al. Mechanisms and kinetics of CO2 hydrogenation to value-added products: a detailed review on current status and future trends. Renew Sustain Energy Rev. 2017;80:1292–311.
Porosoff MD, Yan B, Chen JG. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ Sci. 2016;9(1):62–73.
Müller K, Fleige M, Rachow F, Schmeißer D. Sabatier based CO2-methanation of flue gas emitted by conventional power plants. Energy Proc. 2013;40:240–8.
Erlisa Baraj VS, Hlincink T, Clahoyny K. The Influence of sulphur dioxide on the methanation activity of a nickel based catalyst. Int J Adv Sci Eng Technol. 2016;4:125–8.
Kopyscinski J, Schildhauer TJ, Biollaz SMA. Production of synthetic natural gas (SNG) from coal and dry biomass—a technology review from 1950 to 2009. Fuel. 2010;89(8):1763–83.
Bartholomew CH. Mechanisms of catalyst deactivation. Appl Catal A. 2001;212(1):17–60.
Buttler A, Spliethoff H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review. Renew Sustain Energy Rev. 2018;82:2440–54.
Gahleitner G. Hydrogen from renewable electricity: an international review of power-to-gas pilot plants for stationary applications. Int J Hydrog Energy. 2013;38(5):2039–61.
Götz M, Lefebvre J, Mörs F, McDaniel Koch A, Graf F, Bajohr S, et al. Renewable power-to-gas: a technological and economic review. Renew Energy. 2016;85:1371–90.
Ghaib K, Ben-Fares F-Z. Power-to-methane: a state-of-the-art review. Renew Sustain Energy Rev. 2018;81:433–46.
Sassoye C, Muller G, Debecker DP, Karelovic A, Cassaignon S, Pizarro C, et al. A sustainable aqueous route to highly stable suspensions of monodispersed nano ruthenia. Green Chem. 2011;13(11):3230–7.
Ewald S, Kolbeck M, Kratky T, Wolf M, Hinrichsen O. On the deactivation of Ni-Al catalysts in CO2 methanation. Appl Catal A. 2019;570:376–86.
Karelovic A, Ruiz P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: effect of the metal particle size on catalytic performances and reaction mechanism. Appl Catal B. 2012;113–114:237–49.
Veselovskaya JV, Parunin PD, Okunev AG. Catalytic process for methane production from atmospheric carbon dioxide utilizing renewable energy. Catal Today. 2017;298:117–23.
Veselovskaya JV, Parunin PD, Netskina OV, Kibis LS, Lysikov AI, Okunev AG. Catalytic methanation of carbon dioxide captured from ambient air. Energy. 2018;159:766–73.
Duyar MS, Treviño MAA, Farrauto RJ. Dual function materials for CO2 capture and conversion using renewable H2. Appl Catal B. 2015;168–169:370–6.
Miguel CV, Soria MA, Mendes A, Madeira LM. A sorptive reactor for CO2 capture and conversion to renewable methane. Chem Eng J. 2017;322:590–602.
Veselovskaya JV, Parunin PD, Netskina OV, Okunev AG. A novel process for renewable methane production: combining direct air capture by K2CO3/alumina sorbent with CO2 methanation over Ru/alumina catalyst. Top Catal. 2018;61(15):1528–36.
Duyar MS, Wang S, Arellano-Treviño MA, Farrauto RJ. CO2 utilization with a novel dual function material (DFM) for capture and catalytic conversion to synthetic natural gas: an update. J CO2 Util. 2016;15:65–71.
Wang S, Schrunk ET, Mahajan H, Farrauto JR. The role of ruthenium in CO2 capture and catalytic conversion to fuel by dual function materials (DFM). Catalysts. 2017;7(3):88.
Zheng Q, Farrauto R, Chau Nguyen A. Adsorption and methanation of flue gas CO2 with dual functional catalytic materials: a parametric study. Ind Eng Chem Res. 2016;55(24):6768–76.
Hu L, Urakawa A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/ZrO2. J CO2 Util. 2018;25:323–9.
Janke C, Duyar MS, Hoskins M, Farrauto R. Catalytic and adsorption studies for the hydrogenation of CO2 to methane. Appl Catal B. 2014;152–153:184–91.
Proaño L, Tello E, Arellano-Trevino MA, Wang S, Farrauto RJ, Cobo M. Appl Surf Sci. 2019;479:25
Kim A, Sanchez C, Patriarche G, Ersen O, Moldovan S, Wisnet A, et al. Selective CO2 methanation on Ru/TiO2 catalysts: unravelling the decisive role of the TiO2 support crystal structure. Catal Sci Technol. 2016;6(22):8117–28.
Carenco S, Sassoye C, Faustini M, Eloy P, Debecker DP, Bluhm H, et al. The active state of supported ruthenium oxide nanoparticles during carbon dioxide methanation. J Phys Chem C. 2016;120(28):15354–61.
Debecker DP. Innovative sol-gel routes for the bottom-up preparation of heterogeneous catalysts. Chem Rec. 2018;18(7–8):662–75.
Iarikov DD, Ted Oyama S. Chapter 5—review of CO2/CH4 separation membranes. In: Oyama ST, Stagg-Williams SM, editors. Membrane science and technology. Amsterdam: Elsevier; 2011. p. 91–115.
Zhang Y, Sunarso J, Liu S, Wang R. Current status and development of membranes for CO2/CH4 separation: a review. Int J Greenh Gas Control. 2013;12:84–107.
Tseng H-H, Wang C-T, Zhuang G-L, Uchytil P, Reznickova J, Setnickova K. Enhanced H2/CH4 and H2/CO2 separation by carbon molecular sieve membrane coated on titania modified alumina support: effects of TiO2 intermediate layer preparation variables on interfacial adhesion. J Membr Sci. 2016;510:391–404.
Al-Mamoori A, Rownaghi AA, Rezaei F. Combined capture and utilization of CO2 for syngas production over dual-function materials. ACS Sustain Chem Eng. 2018;6(10):13551–61.
Bobadilla LF, Riesco-García JM, Penelás-Pérez G, Urakawa A. Enabling continuous capture and catalytic conversion of flue gas CO2 to syngas in one process. J CO2 Util. 2016;14:106–11.
Hyakutake T, van Beek W, Urakawa A. Unravelling the nature, evolution and spatial gradients of active species and active sites in the catalyst bed of unpromoted and K/Ba-promoted Cu/Al2O3 during CO2 capture-reduction. J Mater Chem A. 2016;4(18):6878–85.
Sun H, Wang J, Zhao J, Shen B, Shi J, Huang J, et al. Dual functional catalytic materials of Ni over Ce-modified CaO sorbents for integrated CO2 capture and conversion. Appl Catal B. 2019;244:63–75.
Acknowledgements
Paulina Melo Bravo thanks the CONICYT for her PhD fellowship (BECAS CHILE 2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melo Bravo, P., Debecker, D.P. Combining CO2 capture and catalytic conversion to methane. Waste Dispos. Sustain. Energy 1, 53–65 (2019). https://doi.org/10.1007/s42768-019-00004-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-019-00004-0